- Title
-
A New Zebrafish Model for CACNA2D4-Dysfunction
- Authors
- Schlegel, D.K., Glasauer, S.M.K., Mateos, J.M., Barmettler, G., Ziegler, U., Neuhauss, S.C.F.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
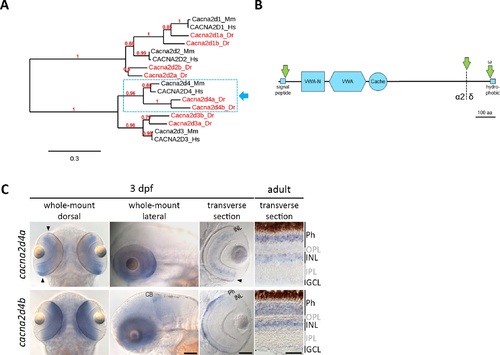
Bioinformatic and expression analysis of the zebrafish orthologues of Cacna2d4. (A) Maximum likelihood phylogenetic tree of the Cacna2d family. Eight members were identified in the zebrafish genome, two of which are clear orthologues of mammalian Cacna2d4 (dashed box). (B) Schematic representation of predicted protein structure applying to both members of the Cacna2d4 subclade (dashed box in A). Cacna2d4 proteins contain Van-Willebrand factor (VWA) and VWA N-terminal (VWA-N) domains, as well as one Cache domain, an N-terminal signal peptide and a ω site adjacent to a short C-terminal hydrophobic region, predicted to mediate proteolytic cleavage and GPI anchoring. Proteolytic cleavage (dashed line) of the Cacna2d4 pro-form gives rise to α2 and δ peptides, which remain attached to each other through disulfide bonds in mature α2δ proteins. (C) In situ hybridization of zebrafish cacna2d4a and cacna2d4b. In 3 dpf zebrafish, cacna2d4a is expressed throughout the retinal INL and in photoreceptors (Phs) of the retinal periphery (arrowheads). In the adult retina, cacna2d4a is expressed in the INL and the distal half of the Ph layer. In larval zebrafish, cacna2d4b shows strong expression in Phs, weak expression in the INL and additional signal in the cerebellum. In the adult retina, cacna2d4b is expressed across the Ph layer and in the INL. Arrows in (B) indicate proteolytic cleavage. aa, amino acids; CB, cerebellum; Dr, Danio rerio; GCL, ganglion cell layer; Hs, Homo sapiens; Mm, Mus musculus. Scale bars correspond to 100 μm in images of 3 dpf zebrafish and 50 μm in adult sections. EXPRESSION / LABELING:
|
|
Cacna1fa localization cacna2d4-KO lines. (A) Immunohistochemical analysis of Cacna1fa with Zpr-1 counterstain (red-green double cones). Cacna1fa expression is not affected in cacna2d4a-KO larvae at 5 and 20 dpf, but severely reduced in cacna2d4b-KO as well as in double-KO. In the latter two, ectopic punctate Cacna1fa staining is observed distal to the OPL (arrowheads). (B) Expression in adult double-KO is comparable to WT levels, yet ectopic puncta are visible (arrowheads). Scale bars (A, B) correspond to 10 μm and apply to all images of the respective developmental stage. |
|
Retinal function of cacna2d4-KO lines at 5 dpf. (A–C) Box and whisker plots of white-light ERG b-wave amplitudes or b-wave kinetics of 5 dpf WT, cacna2d4a-KO, cacna2d4b-KO and double-KO. Bottom and top of the box, the first and third quartile; median, line within the box; whiskers, minimum and maximum values; circles, outliers (1.5 × interquartile distance); small asterisks, extreme outliers (3 × interquartile distance). Five flashes (100 ms) of increasing light intensities were applied, starting from dim light (log-4) to 100% light intensity (log0, 24,000 μW/cm), and responses were grouped accordingly. (A) Neither cacna2d4a nor cacna2d4b-KO disturbed the ERG response at any light intensity (n = 27 per genotype). (B) KO of both paralogs significantly reduced the b-wave amplitude at different light intensities (n = 25 per genotype). (C) The time from baseline to peak is significantly prolonged in double-KO retinae. Significance levels: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (D) Example traces of WT (gray) and double-KO (blue). Yellow bar in (D) represents the stimulus. |
|
Retinal function of cacna2d4-KO lines at 20 dpf. (A–C) Box and whisker plots of white-light ERG b-wave amplitudes, b-wave kinetics and flicker fusion threshold of 20 dpf WT and double-KO. Bottom and top of the box: the first and third quartile; median, line within the box; whiskers, minimum and maximum values; circles, outliers (1.5 × interquartile distance); small asterisks, extreme outliers (3 × interquartile distance). (A, B) Five flashes (100 ms) of increasing light intensities were applied, starting from dim light (log-4) to 100% light intensity (log0, 24,000 μW/cm2), and responses were grouped accordingly (n = 19 per genotype). (A) b-wave amplitude of the mixed rod-cone response was not attenuated in double-KO. (B) The time from baseline to peak is significantly prolonged in double-KO at almost all light intensities. (C) The flicker fusion threshold is reduced in double-KO under photopic conditions (n = 25 per genotype). Significance levels: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. PHENOTYPE:
|
|
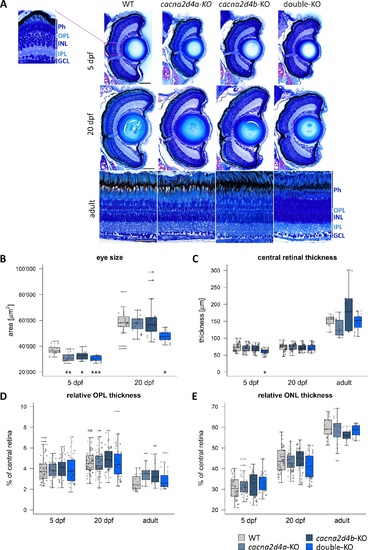
Morphologic analysis of cacna2d4-KO retinae. (A) Semithin plastic sections (3 μm) of WT, cacna2d4a-KO, cacna2d4b-KO and double KO retinae at 5 dpf, 20 dpf, and adult (>5 months). (B–E) Quantification of eye size and retinal layers. Three measurements per section were taken from the central retina, close to the optic nerve. For 5 and 20 dpf fish, eye circumference on central sections was measured as an approximation for eye size. In larvae, both eyes were analyzed, yielding two data points for eye size and six for retinal layers per fish. In adult retinas, two sections per retina and three measurements per section were taken from the central retina, adding to a total of six measurements per fish. 5 dpf: n = 8 each, 20 dpf: WT n = 10; cacna2d4a-KO n = 10; cacna2d4b-KO n = 10; double-KO n = 8, adult: WT n = 4, cacna2d4a-KO n = 3, cacna2d4b-KO n = 3, double-KO n = 4). Box and whisker plots: bottom and top of the box = first and third quartile; the median = line within the box; whiskers = minimum and maximum values; data points with the same shape correspond to the same animal in each box; horizontal lines = outliers. Significance levels: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Scale bars in (A) correspond to 50 μm and apply to all images of the respective developmental stage. |
|
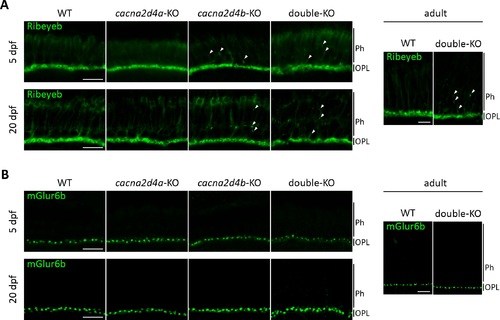
Ribeyeb expression in cacna2d4-KO lines. (A) Ribeyeb expression levels in the OPL of 5 dpf, 20 dpf and adult retina are not affected by mutations in cacna2d4 genes. However, both cacna2d4b-KO and double-KO show ectopic Ribeyeb staining distal to the OPL at all ages examined. (B) mGluR6b expression is comparable to WT in all KO lines. |
|
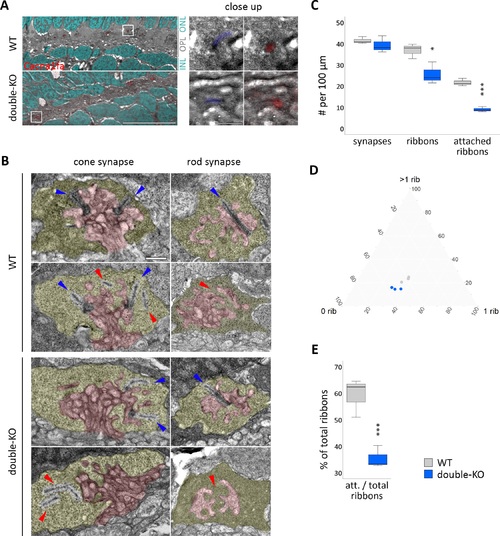
Ultrastructure of the photoreceptor ribbon synapse. (A) Correlative light- and electron microscopy (CLEM) on 5 dpf retinae of WT and double-KO. Cacna1fa puncta seem to be reduced in number in double-KO compared to WT, but both show correct localization of Cacna1fa to synaptic ribbons in the OPL (close up). Red: Cacna1fa antibody staining. Cyan: DAPI. Blue: pseudo-colored ribbon. White frame: close up region. Scale bar in overview corresponds to 10 μm and in close-up to 1 μm. (B) Transmission electron micrographs (TEM) of synapses in 5 dpf WT and double-KO. The presynaptic terminal is pseudocolored in yellow and the postsynaptic invaginations in red. Ribbons that are attached to the active zone are found in cones and rods of both genotypes (blue arrows), but also some that are ‘floating' or missing (red arrows). Scale bar: 0.5 μm. (C–E) Quantification of synapse and ribbon number or attachment on whole-retinal scans of three sections per genotype (one per individual). (C) The number of synapses per 100 μm in double-KO is normal, however the amount of ribbons is reduced and there are fewer ribbons attached to the active zone. (D) Ternary plot of synapse subtypes: 0 rib = empty synapses; 1 rib = synapses with one ribbon; >1 rib = synapses with more than one ribbon; grey = WT; blue = double-KO. The data from WT and double-KO form two separate clusters. The percentage of synapses harboring more than one ribbon (cones) is lower (P = 0.03) and that of empty synapses is higher (P = 0.05) in double-KO. 1-ribbon synapses do not seem to be affected. (E) The number of attached ribbons relative to the total number of ribbons is lower in double-KO retinae. Box and whisker plots: bottom and top of the box, first and third quartile; the median, line within the box; whiskers, minimum and maximum values. Significance levels: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. |