- Title
-
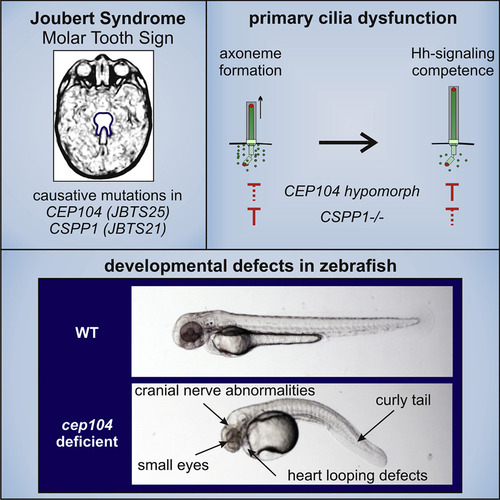
A CEP104-CSPP1 Complex Is Required for Formation of Primary Cilia Competent in Hedgehog Signaling
- Authors
- Frikstad, K.M., Molinari, E., Thoresen, M., Ramsbottom, S.A., Hughes, F., Letteboer, S.J.F., Gilani, S., Schink, K.O., Stokke, T., Geimer, S., Pedersen, L.B., Giles, R.H., Akhmanova, A., Roepman, R., Sayer, J.A., Patzke, S.
- Source
- Full text @ Cell Rep.
|
cep104 Knockdown in Zebrafish Embryos Leads to Ciliopathy Phenotypes (A–C) 48 hpf morphant zebrafish display mild and severe pericardial edema and cardiac defects (∗) following cep104 knockdown and additional phenotypes in severe morphants of mild tail curvature and microopthalmia, with a quantified reduction in area expressed as a ratio to control embryos of 0.45 (p < 0.0001, unpaired t test, n = 39 versus 28 control). (D) Percentage of zebrafish displaying phenotypes following injection of cep104 splice MO and translation blocking morpholino cep104 ATG MO alone or in combination (control n = 98, cep104splice MO n = 166, cep104 ATG MO n = 95, cep104 splice MO + cep104ATG MO n = 77). (E) Western blotting (WB) of cep104 at 48 hpf in zebrafish uninjected and injected with cep104 ATG MO and cep104 splice MO. (F) IFM of cilia and cell junctions (a-acetylated tubulin, red) in Kupffer’s vesicle (KV; atypical protein kinase C [aPKC], green) at the 10-somite stage in control and cep104 knockdown embryos. (G) Dot plots of the length of cilia in KV in control, cep104 splice MO knockdown, and cep104 splice MO and CEP104 mRNA co-injected zebrafish embryos (ANOVA with Tukey post hoc test, ∗p < 0.05). (H) 48 hpf cmlc2:GFP zebrafish treated with cep104 splice MO show changes to heart looping at 48 hpf, which is rescued by co-injection with CEP104 mRNA. (I) Percentage of embryos displaying heart looping phenotypes following injection of cep104 splice MO and co-injection with CEP104 mRNA (∗∗∗p < 0.0001, ∗p = 0.0208, chi-square test of independence; control n = 186; cep104 splicing MO n = 132; cep104splicing MO + CEP104 mRNA n = 130). (J and K) cep104 knockdown in 48 hpf islet-1:GFP transgenic fish leads to cranial nerve defects, rescued by co-injection with CEP104mRNA. Co-injection with CEP104 mRNA produces a partial rescue of phenotypes (∗∗∗p < 0.0001, ∗∗p = 0.0010, chi-square test of independence; control n = 200; cep104 splicing MO n = 80; cep104splicing MO + CEP104 mRNA n = 120). PHENOTYPE:
|
|
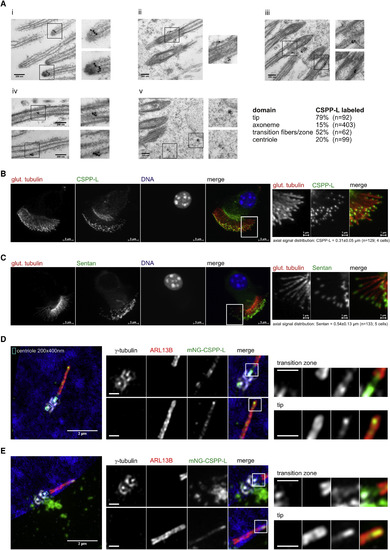
Ciliary Localization of CSPP-L at Motile and Primary Cilia (A) CSPP-L detection by post-embedding IEM of mouse tracheal epithelial cells. Panels depict close ups of (i) cilia tips, (ii and iii) basal bodies, (iv) cilia axonemes, and (v) apically localized electron-dense particles. (B and C) IFM of mouse tracheal epithelial cells showing axonemal MTs (glutamylated tubulin, red) and CSPP-L (B, green) or Sentan (C, green). Right panels show magnifications of indicated regions. (D and E) 3D-SIM IFM of hTERT-RPE1 cells expressing monomeric NeonGreen (mNG)-CSPP-L and co-stained for centrosomal marker γ-tubulin (white) and cilia membrane marker ARL13B (red). Scale bars in magnified areas, 500 nm. mNG-CSPP-L decorates axonemal MTs throughout the transition zone and concentrates at the tip (D and E). Centriolar satellite localization is frequently found and exemplified in (E) and Figure S4C. |
|
|
|
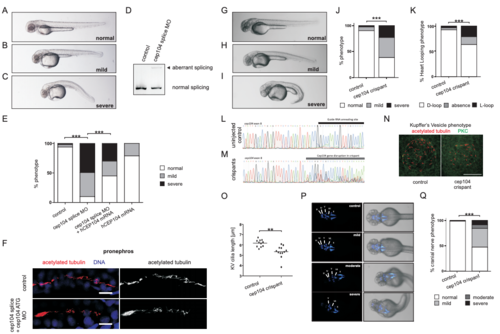
Phenotype of zebrafish treated with a cep104 splice blocking morpholino and ciliopathy phenotypes in cep104 zebrafish crispants (related to Figure 1) (A‐C) 48 hpf zebrafish display pericardial edema and cardiac defects following cep104 splice MO injection, with varying severity from mild (B) to severe (C). (D) Reverse‐transcriptase PCR indicating incorrect splicing (upper band, arrow head) of the cep104 transcript in cep104 splice MO-injected embryos. (E) 48 hpf zebrafish were scored under light microscopy for phenotypes (normal/mild/ severe). Phenotypic rescue of cep104 splice MOinjected embryos with co‐injection of human CEP104 mRNA. Chi square test of independence revealed that the frequencies of phenotypes are significantly different between uninjected controls and morphants (***p<0.0001) and between morphants and morphants co-injected with CEP104 mRNA (***p<0.0001; Control n= 100; cep104 splicing MO n= 150; cep104 splicing MO + CEP104 mRNA n= 160; CEP104 mRNA n=76). (F) IFM of cilia in distal pronephros in control and cep104 morphant embryos at 72 hpf does not reveal an obvious ciliary phenotype in pronephros of cep104 morphant fish. a-acetylated tubulin, red (left panels), white (right panels), DAPI, blue. Scale bars = 10μm. (G-I) Injection of Cas9 protein along with specific guide RNA targeting intron 7/exon 8 of zebrafish cep104 gene results in crispant embryos that phenocopy cep104 morphant embryos, with varying severity from mild (H) to severe (I) when compared to uninjected control (G) at 48 hpf. Crispants exhibited microophthalmia, with a quantified reduction in area expressed as a ratio to control embryos of 0.81, p=0.0038 (unpaired t-test, n = 18 vs. 7 control). Severe crispants and morphants showed some yolk sac abnormalities, which are likely to be linked to pericardial edema. (J) Percentage of control and crispant zebrafish displaying phenotypes. (*** p<0.0001, Chi square test, control n= 229; cep104 crispants n= 240). (K) cep104 crispants display heart looping defects at 48 hpf. Percentage control and crispant fish displaying phenotypes is shown. (*** p<0.0001, Chi square test, control n= 209; cep104 crispants n= 155). (L-M) Sequence chromatograms showing genomic cep104 sequence in an uninjected control embryo (L) and in an embryo injected (M) with Cas9 protein along with specific guide RNA (black bar) targeting cep104. Injection results in genetic mosaic crispant embryos (F0) carrying various mutations in the cep104 gene (M). Mutations in cep104 gene were found in 91% of embryos screened (n= 11). (N,O) IFM of cilia (anti-acetylated tubulin, red) in Kupffer’s Vesicle (KV) at the 10-somite stage in control and cep104 crispant embryos reveals a reduction in cilia length. Cell junctions are stained with anti-PKC (green). Scale bar 50 μm. (O) Dot plots confirming a significant reduction in the length of cilia present in KV in cep104 crispant zebrafish (n=11) when compared to uninjected control (n=12) (** p<0.001, unpaired Student’s t-test). (P) Dorsal views of 48 hpf islet-1:GFP transgenic fish imaged under fluorescence and light microscopy. Injection with Cas9 protein along with sgRNA leads to cranial nerves defects, with varying severity from mild to moderate and severe when compared to uninjected control. (Q) Percentage control and crispant fish displaying phenotypes is shown. (*** p<0.0001, Chi square test, control n= 106; cep104 crispant n= 157).
PHENOTYPE:
|