- Title
-
Elesclomol restores mitochondrial function in genetic models of copper deficiency
- Authors
- Soma, S., Latimer, A.J., Chun, H., Vicary, A.C., Timbalia, S.A., Boulet, A., Rahn, J.J., Chan, S.S.L., Leary, S.C., Kim, B.E., Gitlin, J.D., Gohil, V.M.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
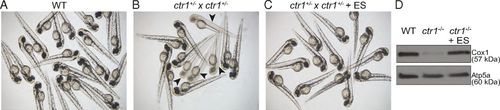
ES supplementation rescues pigmentation and the Cox1 deficiency of ctr1-KO zebrafish. (A–C) Clutches of embryos from a WT zebrafish and from a cross of a pair of ctr1+/− heterozygous zebrafish were imaged at 48 hpf following treatment with and without 10 nM ES. Arrows in B indicate homozygous ctr1−/− embryos with a pigmentation defect. (D) Mitochondria were isolated from 10-dpf WT zebrafish and homozygous ctr1−/− zebrafish treated with and without 10 nM ES. Mitochondrial samples were subjected to SDS/PAGE and immunoblotted using the Complex IV-specific antibody anti-Cox1. The mitochondrial protein Atp5a was used as a loading control. |
|
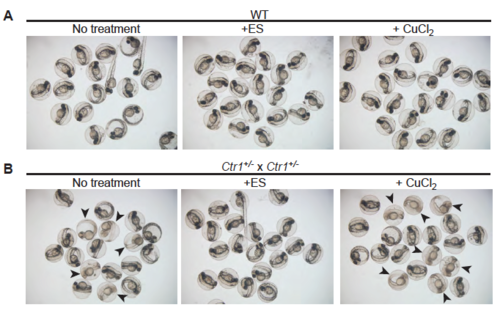
ES supplementation rescues pigmentation of Ctr1 knockout zebrafish while equivalent concentration of copper fails to rescue this defect. (A) Clutches of embryos from a WT zebrafish and (B) from a cross of a pair of Ctr1+/- heterozygous zebrafish were imaged at 48hpf following treatment with and without 100 nM elesclomol (ES) or 100 nM copper chloride (CuCl2). Arrowheads in (B) indicate homozygous Ctr1-/- embryos with a pigmentation defect. Notably, not a single embryo with pigmentation defect was observed upon ES treatment. PHENOTYPE:
|
|
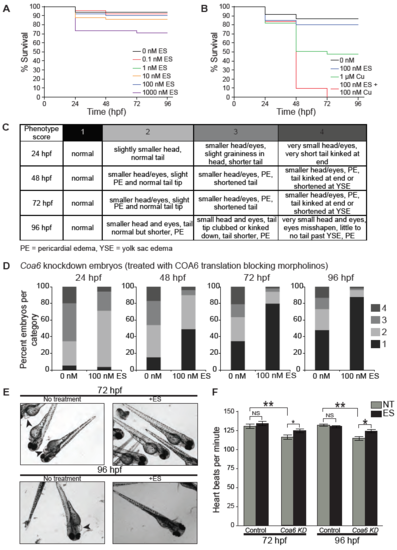
ES treatment improves morphological defects observed in Coa6 knockdown embryos. (A) Zebrafish embryos were treated with indicated concentrations of ES and (B) copper, and combination of both at 3 hours post fertilization (hpf). The surviving fish were counted at the indicated time points. (n ≥30 for each treatment). (C) Table describing phenotype scores (1-4) at different time points in zebrafish embryo development. (D) Phenotypic scores of untreated and ES treated Coa6 knockdown zebrafish embryos at the indicated time points (hpf, hours post fertilization). Batches of 50 fertilized embryos injected with either translation blocking or mismatch control morpholinos were treated with or without 100 nM ES at 3 hpf, before making observations at the indicated time points. (n≥30 for each time point and treatment). (E) Representative images of untreated and ES treated Coa6 knockdown zebrafish embryos at 72 and 96 hpf. The arrow heads indicates cardiac edema. (F) Heart rate of zebrafish embryos treated with either mismatch control morpholino or the Coa6 translation blocking morpholino were measured at the indicated time point. (n≥30 per group; data represent mean ± SEM). |