- Title
-
tbx6l and tbx16 are redundantly required for posterior paraxial mesoderm formation during zebrafish embryogenesis
- Authors
- Morrow, Z.T., Maxwell, A.M., Hoshijima, K., Talbot, J.C., Grunwald, D.J., Amacher, S.L.
- Source
- Full text @ Dev. Dyn.
|
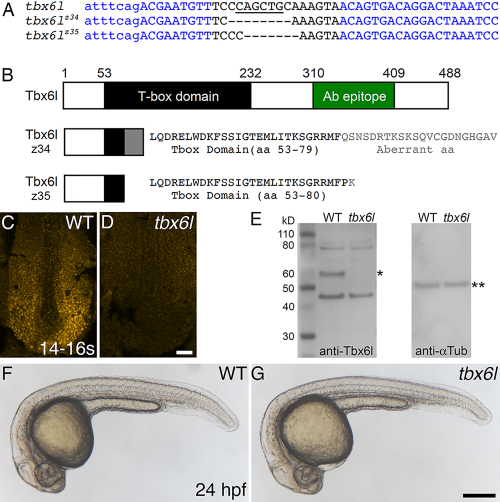
Zebrafish tbx6l mutants do not express Tbx6l protein and are homozygous viable. A: Sequence of tbx6l showing left and right TALEN binding sites (blue) and the spacer region (black) for wild-type, tbx6lz34, and tbx6lz35 alleles. Uppercase letters indicate exon 3 coding sequence; lowercase letters indicate intron sequence. Dashes indicate deleted nucleotides. A PvuII site (underlined) is deleted in both mutant alleles. B: Diagram of wild-type Tbx6l protein and predicted truncated Tbx6l proteins encoded by each mutant allele, showing the T-box domain (black box and black text), aberrant sequence caused by the introduced frameshift in mutant alleles (gray box and gray text), and the immunogen peptide (green box). C,D: Dorsal views of 14- to 16-somite stage wild-type (C) and tbx6lz34 mutant (D) embryos processed by immunofluorescence with Tbx6l antibody. E: Western blot of protein extracts prepared from wild-type (WT) and tbx6z35 homozygous mutant embryos, probed sequentially with anti-Tbx6l antibody (left blot), then with anti-alpha Tubulin antibody as a loading control (right blot). The inferred position of Tbx6l, which runs slightly higher than the predicted 55-kD mass, is marked by a single asterisk, and alpha-Tubulin (50 kD) by double asterisks. F,G: Live images of 24-hpf wild-type (F) and tbx6lz35 mutant (G) embryos. Scale bar in D (for C,D) = 25 μm. Scale bar in G (for F,G) = 250 μm. PHENOTYPE:
|
|
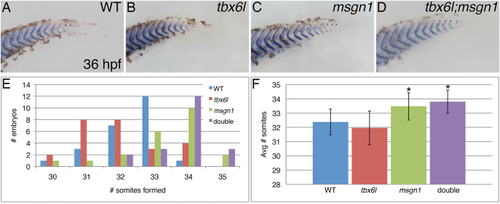
Loss of tbx6l does not enhance the msgn1 mutant phenotype. A–D: In situ hybridization for the cb1045/xirp2a somite boundary marker gene in 36-hpf wild-type (A), tbx6l mutant (B), msgn1 mutant (C), and tbx6l; msgn1 double-mutant (D) embryos that were raised together at standard temperature and fixed several hours after somitogenesis is normally completed. Lateral views of posterior tail are shown. E: Graph showing distribution of somite number per embryo for each genotype. F: Graph showing average somite number per embryo for each genotype. * P < 0.0005, when compared to wild-type. PHENOTYPE:
|
|
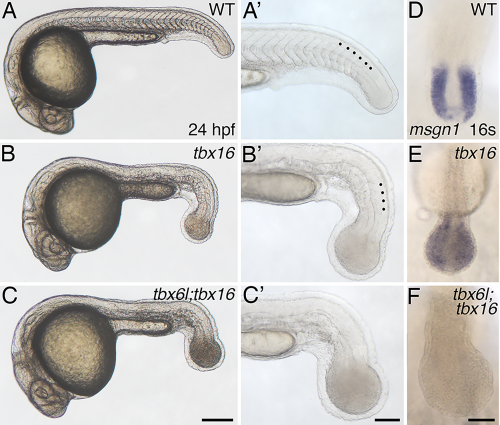
tbx6l; tbx16 double mutants have an enlarged tail bud and lack tail somites. A–C′: Brightfield images of 24-hpf live wild-type (A, A′), tbx16 (spt) mutant (B,B′), and tbx6l; tbx16 double-mutant (C,C′) embryos. tbx6l single-mutant embryos are not shown here as they are indistinguishable from wild-type embryos (see Fig. 1F,G). Black dots in the magnified views (A′–C′) indicate morphologically visible tail somites. D–F: In situ hybridization for msgn1 at the 16-somite stage. Scale bar in A (for A–C) = 250 μm. Scale bar in A′ (for A′–C′) = 100 μm. Scale bar in F (for D–F) = 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
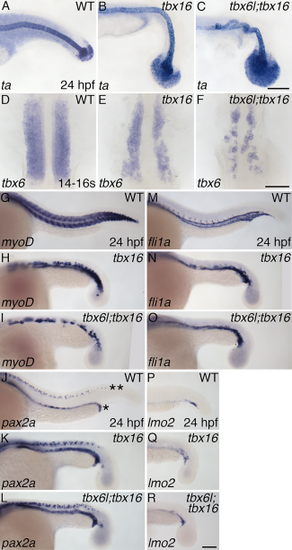
tbx6l and tbx16 have partially redundant roles in formation of tail paraxial mesoderm. A–R: In situ hybridization for ta (A–C), tbx6 (fss) (D–F), myoD (G–I), pax2a (J–L), fli1a (M–O), and lmo2 (P–R) at 24 hpf (A–C;G–R) and the 14- to 16-somite stage (D–F). Not shown are tbx6l single-mutant embryos as they are indistinguishable from wild-type embryos. In panel J, the row of pax2a-expressing pronephros cells is indicated by an asterisk (*), and the row of pax2a-expressing spinal cord neurons is indicated by a double asterisk (**). Scale bars in C (for A–C) and R (for G–R) = 100 μm. Scale bar in F (for D–F) = 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
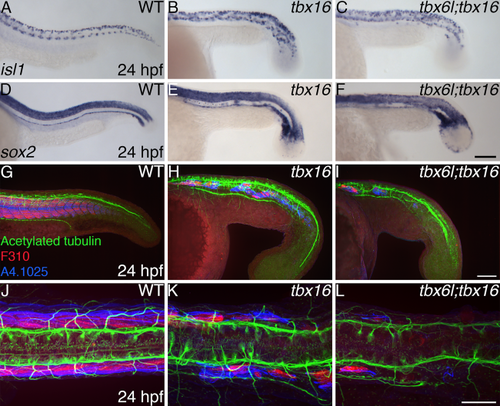
A single neural tube forms in tbx6l; tbx16 double mutants. A–F: In situ hybridization for isl1 (A–C) and sox2 (D–F) at 24 hpf. G–L: Confocal projections of embryos labeled for acetylated tubulin (green), fast muscle (F310; red), and myosin heavy chain (A4.1025; blue) in lateral (G–I) and dorsal (J–L) views. Not shown are tbx6l single-mutant embryos as they are indistinguishable from wild-type embryos. Scale bars in A (for A–F) and G (for G–I) = 100 μm. Scale bar in J (for J–L) = 50 μm. |