- Title
-
The Kinesin Adaptor Calsyntenin-1 Organizes Microtubule Polarity and Regulates Dynamics during Sensory Axon Arbor Development
- Authors
- Lee, T.J., Lee, J.W., Haynes, E.M., Eliceiri, K.W., Halloran, M.C.
- Source
- Full text @ Front. Cell. Neurosci.
|
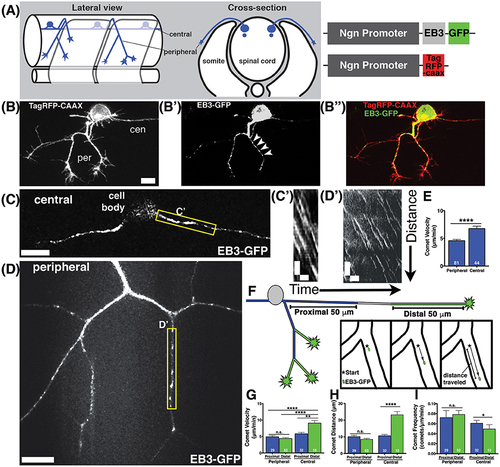
Characterization of microtubule dynamics in sensory neurons. (A) Schematic of RB neurons and DNA constructs with RB promoter (Ngn) driving expression of EB3-GFP or TagRFP-CAAX to label the plasma membrane. (B) RB neurons labeled with TagRFP-CAAX to visualize the peripheral (per) and central (cen) axons. (B') EB3-GFP labels polymerizing MT plus ends. Representative comets are marked with arrowheads. (B”) Overlay of membrane label (red) and EB3-GFP (green). Scale bars are 10 μm in this and all subsequent neuron images. (C,D) RB neurons expressing EB3-GFP in central axons (C) or peripheral axons (D). (C',D') Representative kymographs were made from regions outlined with yellow box in central axons (C') or peripheral axons (D'). All kymographs are oriented with distance on the y axis (μm) and time on the x axis (minutes) with the proximal origin at the top left and the first time point on the left. Scale bars are 5 μm (y) and 1 min (x) for these and all other kymographs. (E) EB3 comets have greater velocity in central axons than peripheral axons (mean peripheral velocity = 4.578 μm/min, n = 81 axon segments; mean central velocity = 6.769 μm/min, n = 44 axon segments; ****p < 0.0001 student's t-test). (F) Schematic showing proximal (blue) and distal (green) regions of central and peripheral axons, with inset box showing how comet distance was measured. (G) EB3 comets in the distal regions of central axons are significantly faster than those in proximal regions (mean distal central velocity = 9.32 μm/min, n = 12 axon segments; mean proximal central velocity = 5.81 μm/min, n = 32 axon segments, **p = 0.0002 student's t-test; mean proximal peripheral velocity = 4.90 μm/min, n = 29 axon segments, ****p < 0.0001 student's t-test; mean distal peripheral velocity = 4.40 μm/min, n = 52 axon segments, ****p < 0.0001 student's t-test). (H) Central axon comets travel further in distal regions than proximal ones (mean proximal central distance = 10.63 μm, n = 32 axon segments; mean distal central distance = 23.20 μm, n = 12 axon segments; ****p < 0.0001 student's t-test). (I) Comet frequency is significantly lower in distal central axon segments than proximal segments (mean proximal central frequency = 0.061 comets/μm/min, n = 32 axon segments, mean distal central frequency = 0.040 comets/μm/min, n = 12 axon segments; *p = 0.045 student's t-test). |
|
Behavior of retrograde comets in wild type axons. (A,B) Wild type neurons expressing EB3-GFP. Small subsets of comets travel retrogradely in peripheral (A) and central (B) axons. Scale bars are 10 μm. (A',B') Time-lapse sequences of representative retrograde comets in yellow boxes. Time is shown in min:sec. (A”,B”) Kymographs of regions outlined by white boxes in (A,B). Retrograde comets can be visualized as positively sloped lines (red) in the kymographs. Scale bars are 5 μm (y) and 1 min (x). (C) The distance retrograde comets travel does not significantly differ from anterograde comets in peripheral or central axons, (mean peripheral anterograde distance = 9.03 μm, n = 81 axon segments, mean peripheral retrograde distance = 7.77 μm, n = axon segments, p = 0.33 student's t-test; mean central anterograde distance = 9.43 μm, n = 44 axon segments, mean central retrograde distance = 8.31 μm, n = 20 axon segments, p = 0.47 student's t-test). (D) Retrograde comet velocities do not differ from anterograde comets. Anterograde comet velocity data is the same as in Figure 1, shown here again for comparison, ****p < 0.0001, student's t-test (mean peripheral retrograde velocity = 5.19 μm/min, n = 18 axon segments; mean central retrograde velocity = 6.91 μm/min, n = 20 axon segments). (E) Retrograde comets traveled similar distances in proximal and distal axon regions (mean peripheral proximal distance = 9.89 μm, n = 7 axon segments, mean peripheral distal distance = 6.42 μm, n = 11 axon segments, p = 0.26 student's t-test; mean central proximal distance = 7.24 μm, n = 12 axon segments, mean central distal distance = 9.90 μm, n = 8 axon segments, p = 0.29 student's t-test). |
|
Clstn-1 loss-of-function (lof) disrupts MT polarity in sensory neuron peripheral axons. (A) In situ hybridization showing clstn-1 mRNA expression in 24 hpf wild type or Clstn-1−/− embryos. (B) qPCR results showing clstn-1 mRNA expression levels normalized to an EF1α positive control (wild type mRNA = −4.681, n = 3 biological replicates of 50 embryos; Clstn-1−/− mRNA = −7.616, n = 2 biological replicates of 50 embryos; *p = 0.014 student's t-test). (C) RB neuron labeled with EB3-GFP in Clstn-1 MO injected embryo. Retrograde comets appear frequently in Clstn-1 lof peripheral axons. Scale bar is 10 μm. (C') Kymograph of the white boxed region in C shows several retrograde comets highlighted in red. Scale bars are 5 μm (y) and 1 min (x). (C”) Time-lapse sequences of the yellow boxed area show a retrograde comet traveling between two branch points (red arrowheads). Time is shown in min:sec. (D) Clstn-1 lof embryos have a higher percentage of retrograde comets than wild type (WT). (WT peripheral = 3.69%, n = 18 neurons; Clstn-1 MO peripheral = 9.29%, n = 18 neurons, †significant via student's t-test comparison to wild type, p = 0.012, but not with Dunnett's post-test after one-way ANOVA p = 0.086; Clstn-1−/− peripheral = 13.55%, n = 17 neurons, ***p = 0.0009 student's t-test, **p = 0.0017 Dunnett's post-test; p = 0.0034 one-way ANOVA). In central axons, there is no significant difference in percentage of retrograde comets between wild type and Cltn-1 lof. (WT central = 9.11%, n = 25 neurons; Clstn-1 MO central = 12.60%, n = 18 neurons, p = 0.27 student's t-test, p = 0.49 Dunnett's post-test; Clstn-1−/− central = 12.10%, n = 16 neurons, p = 0.39 student's t-test, p = 0.61 Dunnett's post-test; one-way ANOVA p = 0.54). (E) Clstn-1−/− neurons have a higher frequency of retrograde comets in peripheral axons than wild type (mean WT peripheral frequency = 0.0039 comets/μm/min, n = 81 axon segments; mean Clstn-1 MO peripheral frequency = 0.0043 comets/μm/min, n = 63 axon segments, p = 0.90 Dunnett's post-test; mean Clstn-1−/− peripheral frequency = 0.012 comets/μm/min, n = 47 axon segments, ***p = 0.0007 Dunnett's post-test; one-way ANOVA ***p = 0.0006). (F) In Clstn-1 lof neurons anterograde comets in central axons travel faster than those in peripheral axons, similar to wild type, (mean Clstn-1 MO peripheral velocity = 5.23 μm/min, n = 63 axon segments, mean Clstn-1 MO central velocity = 7.05 μm/min, n = 26 axon segments, ****p < 0.0001 student's t-test; mean Clstn-1−/− peripheral velocity = 5.53 μm/min, n = 48 axon segments, mean Clstn-1−/− central velocity = 8.74 μm/min, n = 24 axon segments, ****p < 0.0001 student's t-test, wild type velocity data repeated from Figure 1 show here again for comparison). Clstn-1−/− anterograde comets in central axons travel faster than their wild type counterparts (WT vs. Clstn-1−/− mean central velocity *p = 0.031 Dunnett's post-test, WT vs. Clstn-1 MO mean central velocity: p = 0.91 Dunnett's post-test, *p = 0.048 one-way ANOVA). EXPRESSION / LABELING:
PHENOTYPE:
|
|
EB3 comets originate near branch points and growth cones in sensory neuron peripheral axons. (A) RB neuron labeled with EB3-GFP and TagRFP-caax in a Clstn-1 mutant embryo. (A') Yellow box outlines area shown in time-lapse sequence, which shows a retrograde comet originating from a newly forming branch. Scale bar is 10 μm. Time is shown in min:sec. (B) In terminal peripheral axon branches the distance between the origin of retrograde EB3-GFP comets and the growth cone was measured. (C) In non-terminal peripheral axon segments the distance between the origin of retrograde EB3-GFP comets and the distal branch point was measured. (D) Histogram showing the number of retrograde comets originating at indicated distance from the growth cone. (E) Histogram showing the number of retrograde comets originating at indicated distance from branch point. (F) Retrograde comets of Clstn-1 lof originate closer to branch points than wild type (WT mean distance to branch point = 20.26 μm, n = 13 comets; Clstn-1 MO mean distance to branch point = 6.02 μm, n = 18 comets, Dunnett's post-test **p = 0.0030 Dunnett's post-test; Clstn-1−/− near BP distance = 3.55 μm, n = 18 comets, ***p = 0.0005 Dunnett's post-test; ***p = 0.0006 one-way ANOVA). |
|
Clstn-1 is required for stability of filopodia along peripheral axon shafts. (A) Neuron in wild type embryo labeled with TagRFP-caax and EB3-GFP. Stable filopodia structures are often invaded by MTs as seen in the time-lapse sequence in (A'). Time is in min:sec. Scale bar is 10 μm in this and all other neuron images. (B) Neuron in Clstn-1 MO embryo showing collapse of a filopidum in time-lapse sequence in (B'). Filopodia in Clstn-1 lof more frequently collapse, as shown in Clstn-1 MO neuron. Collapsing filopodia usually do not have invading EB3 comets as shown in this montage. (C) Segment of peripheral axon labeled with TagRFP-caax and EB3-GFP. EB3-GFP signal brightness is increased to show fainter signal in filopodia. Filopodia labeled 1, 2, and 3 are shown in time-lapse in (C1–C3). Time-lapse sequences showing stable filopodia containing static EB3-GFP accumulations at their distal tips over several minutes in (C1,C2), while collapsing filopodia have no EB3 accumulations, as seen in C3. (D) A greater percentage of filopodia collapse in Clstn-1 lof than in wild type (WT collapse = 26.11% in n = 16 neurons, Clstn-1 MO collapse = 53.62% in n = 9 neurons, *p = 0.039 Dunnett's post-test, Clstn-1−/− collapse = 55.83% in n = 10 neurons, *p = 0.020 Dunnett's post-test, *p = 0.0145 one-way ANOVA). (E) There is no significant difference in the rate of EB3-GFP invasions into filopodia between wild type and Clstn-1 lof (mean WT rate = 0.124 comets/min, n = 14 neurons; mean Clstn-1 MO rate = 0.0885 comets/min, n = 8 neurons; mean Clstn-1−/− rate = 0.0724 comets/min, n = 8 neurons; p = 0.068 one-way ANOVA). (F) Percentages of filopodia not invaded by MTs that collapse or remain stable during a 3-min period. (WT = 28.2%, n = 71 filopodia; Clstn-1 MO = 63.6%, n = 33 filopodia, ***p = 0.0003 chi-squared test; Clstn-1−/− = 52.5%, n = 40 filopodia, ***p = 0.0002 chi-squared test). PHENOTYPE:
|