- Title
-
Paxillin Genes and Actomyosin Contractility Regulate Myotome Morphogenesis in Zebrafish
- Authors
- Jacob, A.E., Amack, J.D., Turner, C.E.
- Source
- Full text @ Dev. Biol.
|
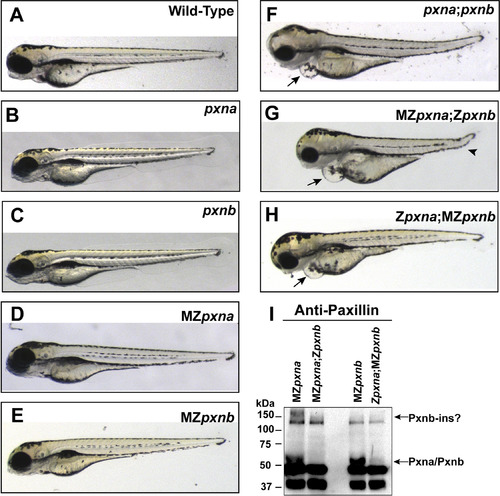
Double Paxillin mutant zebrafish embryos develop gross morphological defects. (A-H) Gross morphology of wild-type (A), homozygous zygotic single pxn mutant (B, C), maternal and zygotic (MZ) single pxn mutant (D, E), zygotic double pxn mutant (F) and MZ double pxn mutant (G, H) embryos at 4 dpf. Defects in double mutants included pericardial edema (arrows in F, G, H), and kinked tail (arrowhead in G). (I) Western blotting of lysates from 4 dpf embryos revealed that double pxn mutants lacked full-length Pxna and Pxnb proteins, including a high molecular weight band possibly corresponding to the Pxnb-ins isoform. |
|
Maternal Paxillin expression differentially regulates cardiovascular and notochord development. (A) Gross morphological examination of the head and pericardial regions revealed that pxn double mutant embryos lacking maternal pxna expression (MZpxna;Zpxnb) exhibited swelling near the brain (arrowhead) and heart (arrow) at an earlier time point than those lacking maternal pxnb (Zpxna;MZpxnb). The number of MZ double mutants exhibiting the depicted phenotype over the total number of double mutants analyzed for each stage is shown. (B) 3D surface projections of Myosin immunostaining of the developing heart at 48 hpf or 3 dpf in MZpxna;Zpxnb and Zpxna;MZpxnb embryos. Disrupted cardiac morphology was observed in both pxn MZ double mutant genotypes. The number of hearts observed with the depicted phenotypes over the total number of embryos analyzed for each genotype is shown. Scale bars=50 µm. (C) At 48 hpf, outer notochord sheath cells in the tail of the embryo (boxed region of embryo diagram) normally exhibit punctate ZO-1 distribution at cell-cell junctions in sagittal optical sections (arrows) as observed in MZpxna, MZpxnb and Zpxna:MZpxnb embryos. A portion of MZpxna;Zpxnb embryos exhibited sheath cell rounding and extrusion from the monolayer with disrupted ZO-1 distributions (arrowhead). The number of notochords observed with the depicted phenotypes over the total number of embryos analyzed for each genotype is shown. Scale bar=10 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
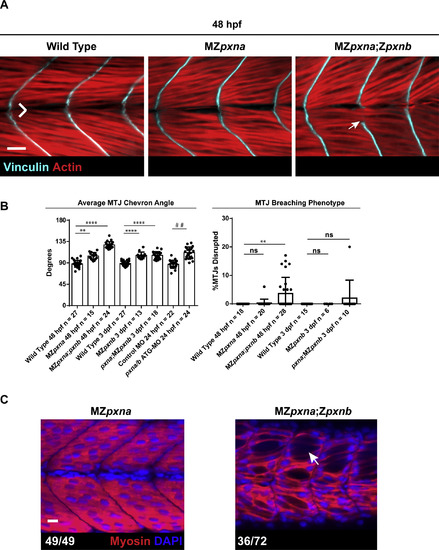
Paxillin mutants exhibit defects in myotome development. (A) Vinculin and Actin were visualized in 4 dpf embryos to show MTJs and myofibers respectively. Chevron angle (B) was measured for three consecutive MTJs and averaged for each embryo. MZpxna;Zpxnb embryos exhibited over-elongated myofibers passing through MTJs (white arrow) in some of their myotomes. Myofiber detachment and retraction (yellow arrowhead) was also observed. Zpxna;MZpxnb embryos had more mild MTJ breaching defects at this stage. Scale bar=10 µm (B) MTJ chevron angles and disrupted MTJs were quantified at 4 dpf in wild-type embryos, Paxillin MZ single mutants, Paxillin MZ double mutants and wild-type embryos injected with either control MO or pxna/b ATG-MO. Ectopic expression of GFP-Pxna fusion protein partially rescued myotome defects in pxna/b ATG-MO injected embryos. Data points represent individual embryo means and error bars show standard deviations from three independent experiments. **** p<0.001 as determined by ANOVA/Fisher's LSD post-hoc test, # # p<0.01 as determined by T-test, ns=not significant. |
|
Paxillin regulates ECM composition at the MTJ. (A) Immunostaining for Laminin and Vinculin at MTJs at 24 hpf in wild-type, MZpxna, MZpxna;Zpxnb, MZpxnb, Zpxna;MZpxnb and control MO or pxna/b ATG-MO injected wild-type embryos. (B) Mean fluorescence intensity (MFI) ratios between Laminin and Vinculin revealed reduced Laminin accumulation at the MTJs of MZpxna;Zpxnb embryos and pxna/b ATG-MO injected embryos at this stage. Data points represent individual embryo means and error bars show standard deviations from three independent experiments. * p<0.05 as determined by Kruskal-Wallis/Dunn's post-hoc test, # # p<0.01 as determined by T-test, ns = not significant. (C) Immunostaining for Fibronectin at MTJs at 32 hpf. Ectopic retention of Fibronectin at MTJs in the majority of MZpxna;Zpxnb embryos was observed (white arrowheads). The number of embryos from three independent experiments with the depicted MTJ phenotypes over the total number of embryos examined for each genotype is noted. (D) Immunostaining for Fibronectin at MTJs at 48 hpf. Similar to MZpxna embryos, Fibronectin gets downregulated at the MTJs in a majority of MZpxna;Zpxnb embryos. Some MZpxna;Zpxnb embryos with severe muscle degeneration exhibit persistent ectopic Fibronectin (white arrowhead). The number of embryos exhibiting each phenotype over the total number of embryos examined from three independent experiments is noted. Boxed areas show regions of interest visualized for each stage. Scale bars =10 µm. |
|
Localization of NMM-II to somite boundaries precedes Pxna recruitment. Montage of live imaging of somite boundary formation at 18 hpf in the tail (boxed region of embryo diagram) of double transgenic embryos that express Myl12.1-mKate2 and Pxna-GFP fusion proteins. Arrowheads depict forming somite boundaries in each channel. EXPRESSION / LABELING:
|
|
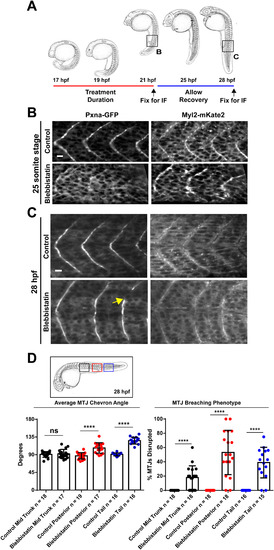
NMM-II Activity Shapes the Developing Myotome. (A) Diagram depicting the timing of pharmacological treatments with blebbistatin to inhibit NMM-II and the immunofluorescence (IF) staining of MTJ markers. Regions visualized by IF are boxed. (B) Embryos treated with blebbistatin formed MTJs marked by Myl12.1-mKate2, but Pxna-GFP MTJ localization was reduced as compared to control embryos at the 25 somite stage (C) Embryos treated with blebbistatin that were washed and allowed to develop without the drug recovered Pxna-GFP localization to MTJs by 28 hpf. However, wider chevron angle and MTJ gaps (yellow arrow) were observed. (D) Width of MTJ angle and gaps in MTJs were quantified in blebbistatin treated and control embryos at 28 hpf in the anterior region of the trunk, the posterior region of the trunk and the tail (see boxed region of embryo diagram). Data points represent individual embryo means and horizontal bars show means for each complete dataset from three independent experiments. **** p<0.001, ns = not significant. Scale bars =25 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cardiac Left-Right Patterning and Sarcomere Assembly are Unaffected in Paxillin MZ Double Mutants. (A) Whole-mount mRNA in situ hybridization for heart-specific cmlc2 expression at the heart-jogging stage (30 hpf) revealed that asymmetric heart patterning is normal in Paxillin MZ double mutant embryos (indicated by direction of arrows). The number of embryos with proper heart-jogging direction over total number examined is indicated for each genotype. (B) Immunostaining for α-Actinin revealed that sarcomere formation is also normal in Paxillin MZ double mutant embryos. The number of embryos with normal Z-disc organization over total number examined is indicated for each genotype. |
|
Myotome and MTJ Defects Develop by 48 hpf in MZpxna;Zpxnb embryos. (A) Immunostaining for myofibers and MTJs at 48 hpf revealed that MZpxna;Zpxnb embryos exhibit large gaps and myoblast elongation through MTJs (white arrow). Scale bar =25 µm (B) Quantification of MTJ chevron angle and MTJ breaching revealed that MTJ defects arise by 48 hpf in Paxillin MZ single and double mutant embryos. Injection of the pxna/b ATG-MO also resulted in a wider MTJ chevron angle compared to control MO injected embryos at an earlier stage of development. Data points represent individual embryo means and error bars show standard deviations from three independent experiments. **** p<0.001, ** p<0.005 determined by Kruskal-Wallis test with Dunn's multiple comparisons post-hoc test, # # p<0.01 determined by T-test, ns = not significant. (C) Immunostaining for Myosin revealed that a substantial number of MZpxna;Zpxnb embryos had large gaps between myofibers (arrow). The number of embryos exhibiting depicted phenotypes over the total number of embryos examined for three independent experiments is shown. Scale bar =10 µm. |
|
Validation of pxna/b ATG-MO Efficacy by Western Blotting and Rescue Experiments. (A) Targeting diagram for pxna/b ATG-MO. Sequence similarity between exon 1 (E1) and exon 2 (E2) of pxna and pxnb transcripts allows for translation blocking of both genes using one MO. Alignment shows MO target sequence compared with pxna and pxnb transcripts. Yellow highlights nucleotides shared between all sequences while blue highlights those shared between two sequences. (B) Western blotting with Paxillin antibody revealed robust knockdown of endogenous Pxn proteins in embryos injected with pxna/b ATG-MO and expression of exogenous GFP-Pxna fusion protein in embryos injected with pxna/b ATG-MO + GFP-Pxna mRNA at 3 dpf. (C) Pxn protein knockdown resulted in myotome defects which included wide MTJ chevron angles and MTJ breaches (arrows), these phenotypes were partially rescued by GFP-Pxna expression that localized to MTJs. Scale bar =50 µm. |
Reprinted from Developmental Biology, 425(1), Jacob, A.E., Amack, J.D., Turner, C.E., Paxillin Genes and Actomyosin Contractility Regulate Myotome Morphogenesis in Zebrafish, 70-84, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.