- Title
-
Vegfd modulates both angiogenesis and lymphangiogenesis during zebrafish embryonic development
- Authors
- Bower, N.I., Vogrin, A.J., Le Guen, L., Chen, H., Stacker, S.A., Achen, M.G., Hogan, B.M.
- Source
- Full text @ Development
|
vegfd regulates facial lymphangiogenesis. (A) Forward (red) and reverse (blue) Talens used to target the vegfd locus induced a 7 bp deletion designated as the uq9bh allele. (B) The uq9bh allele results in a premature stop codon at amino acid 65, prior to the VEGF homology domain. (C,D) Confocal images of Tg(-5.2lyve1b:dsRed);Tg(flt1:yfp) wild-type (C, lyve1b:dsRed only; C′) and vegfduq9bh mutant embryos (D, lyve1b:dsRed only; D′) at 5 dpf. (E-H) vegfduq9bh adult mutants are viable and have no obvious defects (G,H) when compared with siblings (E,F). (J-N′) Confocal images showing the facial region of 5 dpf Tg(fli1a:negfp);Tg(-5.2lyve1b:dsRed) transgenic embryos produced by in-crossing vegfduq9bh, vegfchu5055 double heterozygotes. Arrows indicate the presence of normal medial facial lymphatics (MFL), lateral facial lymphatics (LFL) and otolithic lymphatic vessel (OLV). Asterisks indicate the reduction of LEC number in these vessels. (O-Q) vegfduq9bh mutant embryos have a reduced number of facial lymphatic endothelial cells (LECs) when quantified in 5 dpf Tg(fli1a:negfp):Tg(-5.2lyve1b:dsRed) embryos. (O) Significant differences in LFL LEC numbers were observed when vegfduq9bh mutants (n=5) were compared with vegfduq9bh heterozygous (n=12) or wild-type (n=7) embryos in a vegfchu5055 wild-type background; and when vegfduq9bh mutants (n=7) were compared with vegfduq9bh heterozygous (n=18) or wild-type (n=6) embryos in vegfchu5055 mutants. (P) Significant differences in MFL LEC numbers were observed when comparing vegfduq9bh mutants (n=6) with vegfduq9bh wild-type (n=7) embryos in a vegfchu5055 wild-type background; when comparing vegfduq9bh mutant (n=10) or heterozygous embryos (n=18) with vegfduq9bh wild type (n=6) and embryos in a vegfchu5055 heterozygous background; and when vegfduq9bh mutants (n=9) were compared with vegfduq9bh heterozygous (n=18) and wild-type (n=6) embryos in a vegfchu5055 mutant background. (Q) There was no interaction between vegfc and vegfd in the formation of the otolithic lymphatic vessel. Data represent mean±s.e.m.; ***P<0.001, ** P<0.01, *P<0.05 from one-way ANOVA from three independent clutches of embryos. |
|
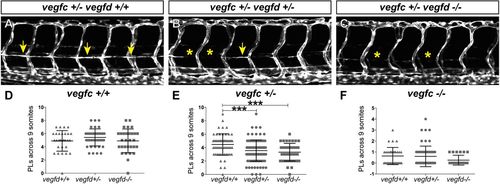
Vegfd contributes to the development of parachordal lymphatic endothelial cells. (A-C) Confocal images at 54 hpf showing presence (arrows) or absence (asterisks) of PL formation in representative Tg(fli1a:egfp) vegfchu5055, vegfduq9bh mutant embryos. (D-F) Quantification of PL formation in vegfduq9bh mutant embryos in vegfchu5055 wild-type (D), heterozygous (E) and mutant (F) backgrounds. There were significantly fewer PLs formed in vegfduq9bh homozygous mutant (n=42) and heterozygous (n=112) embryos when compared with vegfduq9bh wild-type (n=63) embryos in a vegfchu5055 heterozygous background (E). Error bars represent mean±s.e.m.; ***P<0.001 from one-way ANOVA from three independent clutches of embryos. |
|
Vegfd contributes to thoracic duct development in the zebrafish trunk. (A-F) Confocal images of Tg(fli1a:egfp) at 5 dpf showing formation of the TD (arrows) is normal in vegfduq9b mutants (A,B). The phenotype for the formation of the TD is variable in vegfchu5055 mutants, ranging from a complete loss (C) to very mild loss (D). The formation of the TD is reduced (asterisk) in vegfchu5055 mutants that were heterozygous (E) or mutant (F) for vegfduq9bh. (G-I) Quantification of TD extent across nine somites in vegfduq9bh mutant embryos in vegfchu5055 wild-type (G), heterozygous (H) and mutant (I) backgrounds. There was significantly less TD formed in vegfduq9bh mutant embryos (n=42) when compared with vegfduq9bh heterozygous (n=112) and wild-type (n=63) embryos in a vegfchu5055 heterozygous background (H); and in vegfduq9bh mutant embryos (n=25) when compared with vegfduq9bh heterozygous (n=56) and wild-type (n=33) embryos in a vegfchu5055 homozygous mutant background (I). Data represent mean±s.e.m.; *P<0.05 from Kruskal–Wallis test from three independent clutches of embryos. (J-K′) Confocal images of TD formation in 5 dpf Tg(fli1a:negfp),Tg(-5.2lyve1b:dsRed) embryos showing that fragments of TD form in vegfchu5055 mutant/vegfduq9bh wild-type embryos (J,J′), but are absent in vegfchu5055 /vegfduq9bh double-mutant embryos (K,K′). (L) Quantification of experiment in J-K′. The number of nuclei present in the TD of vegfduq9bh mutant (n=14) embryos was significantly lower when compared with vegfduq9bh heterozygous (n=21) and vegfduq9bh wild-type (n=13) embryos in a vegfchu5055 mutant background. Data represent mean±s.e.m.; **P<0.01, from one-way ANOVA from three independent clutches of embryos. (M,N) Confocal images of TD formation in 7 dpf Tg(-5.2lyve1b:dsRed) embryos (yellow arrows) in a flt4hu4602 heterozygous background. vegfduq9bh mutants (N) have reduced TD formation compared with vegfduq9bh wild-type embryos (M). (O) Quantification of experiment in M,N. There was significantly less TD formed in vegfduq9bh mutant embryos (n=33) when compared with vegfduq9bh heterozygous (n=19) and wild-type embryos (n=27) in a flt4hu4602 heterozygous background. **P<0.01, *P<0.05 from Kruskal–Wallis test from three independent clutches of embryos. |
|
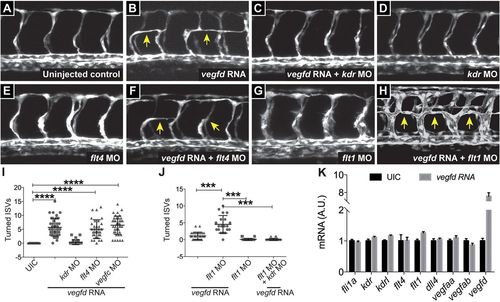
Vegfd mRNA injection induces artery defects in a Kdr-dependent manner. (A-F) Injection of vegfd RNA into Tg(fli1a:egfp) embryos causes bilateral turning of aISVs (arrows) at 28-32 hpf (n=31, A,B), which is rescued by co-injection of a morpholino against kdr (C,D), but not flt4 morpholino injection (E,F). (G,H) Co-injection of flt1 morpholino and vegfd RNA increases the bilateral turning of aISVs (n=20) when compared with vegfd RNA (n=31) or flt1 morpholino alone (n=29) (compare A,B,G and H). (I,J) Quantification of experiments presented in A-E (I) and G,H (J). Data represent mean±s.e.m.; ****P<0.0001, ***P<0.001 from one-way ANOVA. Data were obtained from three independent injections. (K) qPCR analysis of vegf receptor and ligand expression in uninjected and vegfd mRNA-injected embryos shows no significant difference in mRNA levels for any of the genes examined. |
|
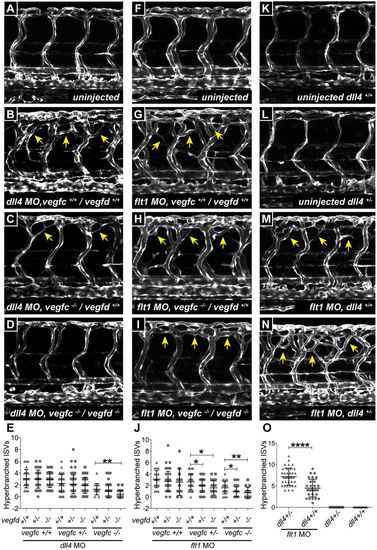
Dll4 and Flt1 suppress Vegfc and Vegfd signaling in developing intersegmental arteries. (A-D) Confocal images of aISVs in 3 dpf Tg(fli1a:egfp) uninjected wild-type control (A), dll4 morpholino (MO)-injected control (B), dll4 MO-injected vegfchu5055 mutant (C) and dll4 MO-injected vegfchu5055, vegfduq9bh double mutant (D) embryos. Arrows indicate hyperbranching. (E) Quantification of hyperbranching in the experiment shown in A-D. dll4 MO-injected vegfchu5055, vegfduq9bh double-mutant embryos (n=20) had significantly fewer hyperbranched aISVs than dll4 MO-injected vegfchu5055 mutants (n=15). (F-I) Confocal images of aISVs in 3 dpf Tg(fli1a:egfp) uninjected wild-type control (F), flt1 MO-injected control (G), flt1 MO-injected vegfchu5055 mutant (H) and flt1 MO-injected vegfchu5055, vegfduq9bh double-mutant embryos (I). Arrows indicate hyperbranching. (J) Quantification of hyperbranching in the experiment shown in F-I. flt1 MO-injected vegfchu5055, vegfduq9bh double-mutant embryos (n=34) had significantly fewer hyperbranched aISVs than vegfduq9bh heterozygous (n=75) and wild-type (n=43) embryos in a vegfchu5055 heterozygous background. vegfduq9bh mutant embryos (n=14) also had significantly fewer hyperbranched aISVs than vegfduq9bh heterozygous (n=52) and wild-type (n=23) embryos in a vegfchu5055 mutant background. (K-N) Confocal images of aISVs in uninjected Tg(fli1a:egfp) wild-type embryos (n=21) (K), uninjected dll4uq10bh heterozygous embryos (n=22) (L), flt1 MO-injected wild-type embryos (n=37) (M) and flt1 MO-injected dll4uq10bh heterozygous embryos (n=29) (N) at 3 dpf. Arrows indicate hyperbranching. (O) Quantification of hyperbranching in the experiment shown in K-N. There was no hyperbranching of aISVs in dll4uq10b heterozygous embryos compared with their wild-type siblings at 3 dpf (the phenotype initiates later in heterozygotes – see Fig. S3C). Hyperbranching was induced by 3 dpf following injection of flt1 MO in wild-type embryos and the effect was significantly enhanced in dll4uq10b heterozygous embryos. For all of the above analysis, data are mean±s.e.m.; ****P<0.0001, **P<0.01, *P<0.05 from one-way ANOVA. Data were obtained from three independent MO injections. EXPRESSION / LABELING:
PHENOTYPE:
|
|
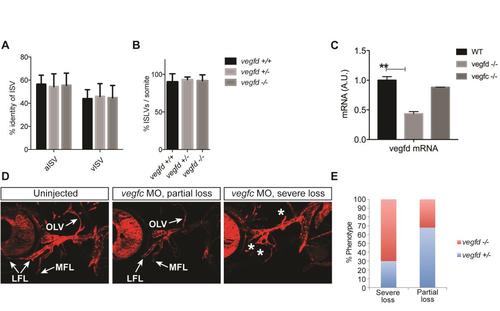
Characterisation and quantification of vessels in vegfduq9bh mutant embryos. (A) Quantification of the percentage of arterial intersegmental vessels (aISVs) and venous intersegmental vessels (vISVs) at 5dpf per somite across 10 somites anterior from the end of the yolk extension in vegfduq9bh heterozygous, mutant and wildtype embryos reveals no significant differences between genotypes. Error bars represent mean +/- sem; one way ANOVA analysis (n=6). (B) Quantification of the percentage of ISLVs at 5dpf per somite across 10 somites anterior from the end of the yolk extension in vegfduq9bh heterozygous, mutant and wildtype embryos reveals no significant differences between genotypes. Error bars represent mean +/- sem; one way ANOVA analysis (n=6). (C) Analysis of vegfd mRNA levels in embryos derived from an incross of vegfd adult mutants showing mutant embryos had approximately 50% of the transcript levels of wildtype embryos, suggesting non-sense mediated decay of mutant transcripts There was no change in vegfc mRNA levels in vegfduq9bh mutants indicating that compensation for the loss of vegfd does not occur in zebrafish. (D) Embryos from a vegfduq9bh heterozygote x vegfduq9bh homozygous mutant cross, injected with vegfc morpholino, were characterised for the loss of facial lymphatics as severe, moderate or none. (E) Subsequent genotyping found that the vegfduq9bh mutant embryos were enriched in the severe loss of facial lymphatic category. |
|
The bilateral turning of aISVs following vegfc RNA injection is rescued by flt4 morpholino injection. (A) Injection of vegfc RNA causes bilateral turning of aISVs at 28-32hpf, which is rescued by co-injection of a flt4 morpholino as previously described (Hogan et al., 2009b). (B) Quantification of turned aISVs in embryos that were uninjected (n=35), injected with vegfc RNA (n=29), or vegfc RNA co-injected with kdrl (n=23), kdr (n=27) and flt4 (n=30) morpholinos. These experiments were performed in parallel with the vegfd mRNA experiments reported in Figure 4. |
|
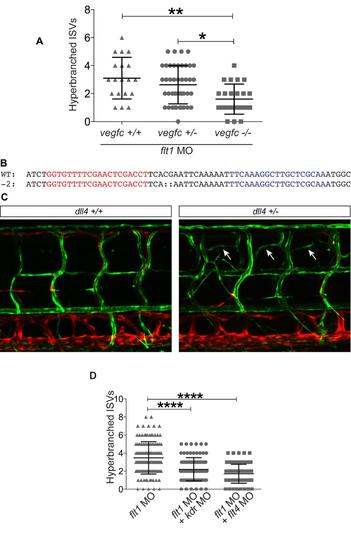
Additional data related to Figure 6 (A) Quantification of artery hyperbranching in wildtype (n=20), vegfchu5055 heterozygous (n=43) and homozygous (n=23) embryos injected with a flt1 morpholino (MO). There are significantly fewer hyperbranched aISVs in vegfc heterozygous and mutant embryos when compared with vegfc wildtype embryos. (B) Sequence details for Talen induced mutagenesis. Talen induced mutagenesis by injection of forward and reverse Talens targeting the red and blue regions of the dll4 locus. Mutagenesis induced a 2bp deletion in the exon2 of the dll4 coding sequence (dll4uq10bh), predicted to generate a null allele due to the introduction of a stop codon at amino acid 47. (C) Confocal images of the trunk of 5dpf Tg(flt11:yfp);Tg(-5.2lyve1b:dsRed) zebrafish embryos showing hyperbranching (arrows) of aISVs in dll4uq10bh heterozygous embryos, which is absent in wildtype embryos. (D) The hyperbranching of aISVs at 3dpf following injection of flt1 MO (n=128) is partially rescued by co-injection with either kdr (n=120) or flt4 MO (n=91). For above analysis, error bars represent mean +/- sem; ****p<0.0001, ***p<0.001, ** p<0.01, *p<0.05 from one way ANOVA. |