- Title
-
Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart
- Authors
- Cavanaugh, A.M., Huang, J., Chen, J.N.
- Source
- Full text @ Dev. Biol.
|
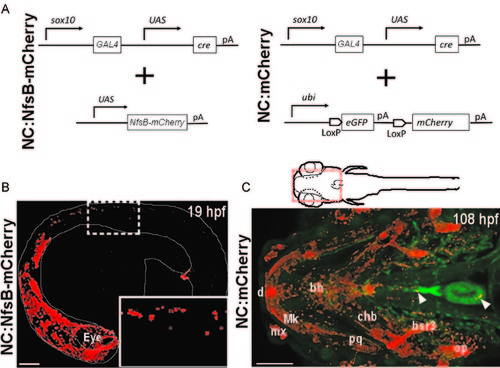
sox10 Transgenic constructs and expression in zebrafish. (A) Schematic representation of transgenic strategy used to generate NC:NfsB-mCherry and NC:mCherry lines. (B) Confocal image of NC:NfsB-mCherry fish at 19 hpf from a lateral view (anterior to left). The mCherry positive cells are found in cranial and trunk neural crest (box enlarged in inset). (C) Ventral view of the anterior region (indicated by red box on illustration) of NC:mCherry; kdrl:GFP embryo at 108 hpf (anterior to left). The mCherry positive cells are observed in craniofacial structures, as well as the heart (arrowhead) and around the ventral aorta (arrowhead). bh, basihyal; bsr3, branchiostegal ray 3; chb, ceratohyal; d, dentea; mx, maxilla; Mk, Meckel′s cartilage; op, opericle; pq, palatoquadrate. Scale bars=100 µm. |
|
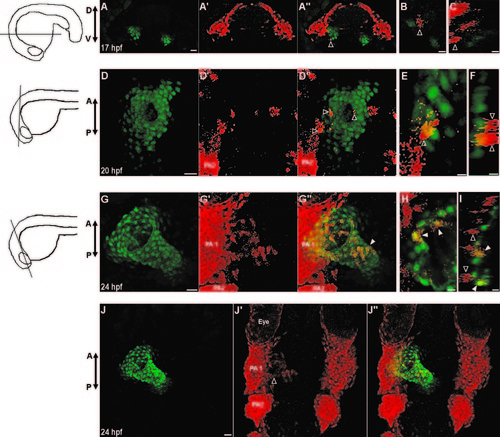
Neural crest cells contribute to the primitive heart tube. (A–J) Confocal images of vibratome sections of NC:NfsB-mCherry; myl7:nucGFP embryos. Orientation of each section is indicated by illustration on the left. Sox10 positive cells are marked by mCherry and myl7 positive cardiomyocytes express nuclear GFP. At 17 hpf (A–C) and 20 hpf (D–F) mCherry+ cells are in close proximity of the heart and have not adopted a myocardial fate (open arrowhead). (G–I) At 24 hpf, some mCherry+ cells have integrated into the primitive heart tube and express nucGFP (closed arrowhead) while those mCherry+ cells adjacent to the heart do not (open arrowheads). (J–J′′) Dorsal view of the anterior region of the same embryo in G, showing mCherry+ cells migrating out of PA1 (open arrowhead). (A, D, G, J) Confocal projections of vibrotome sections, scale bar=20 µm. (B, E, H) Single confocal section, scale bar=5 µm. (C, F, I) Orthogonal reconstruction, scale bar=5 µm. PA1, pharyngeal arch 1; PA2, pharyngeal arch 2, D, dorsal; V, ventral; A, anterior; P, posterior. |
|
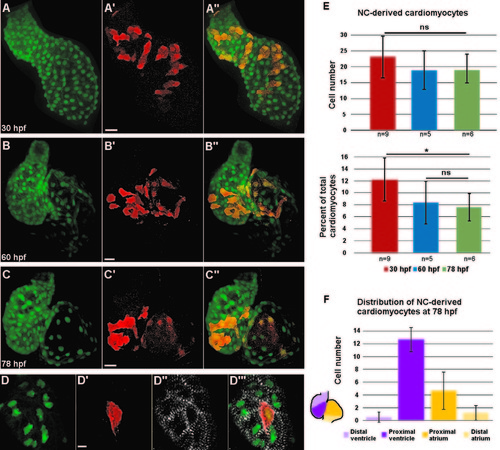
Distribution of neural crest-derived cardiomyocytes in developing zebrafish heart. (A–C′′) Confocal projections of NC:NfsB-mCherry; myl7:nucGFP embryonic hearts at 30 hpf (A–A′′), 60 hpf (B–B′′), and 78 hpf (C–C′′). (A–A′′) NC-derived mCherry positive cells are distributed along the primitive heart tube at 30 hpf. (B–C′′) As development progresses, mCherry+ cells become excluded from the poles of the heart. Scale bar=20 µm. (D–D′′′) Confocal image of the ventricle of a NC:NfsB-mCherry; myl7:nucGFP embryo at 72 hpf. Immunostaining for α-actinin (white) demonstrates well-developed sarcomeres in NC-derived cardiomyocytes. Scale bar=5 µm. (E) Quantification of the number (top) and the percentage (lower panel) of NC-derived cardiomyocytes (positive for both mCherry and nucGFP) at 30 hpf, 60 hpf and 78 hpf. (F) At 78 hpf NC-derived cardiomyocytes contribute primarily to the proximal ventricle (12.7±1.9) and proximal atrium (4.7±2.9), with very little contribution to the distal ventricle (0.5±0.8) and distal atrium (1.2±1.5) (n=6). *p<0.05; ns, not significant. |
|
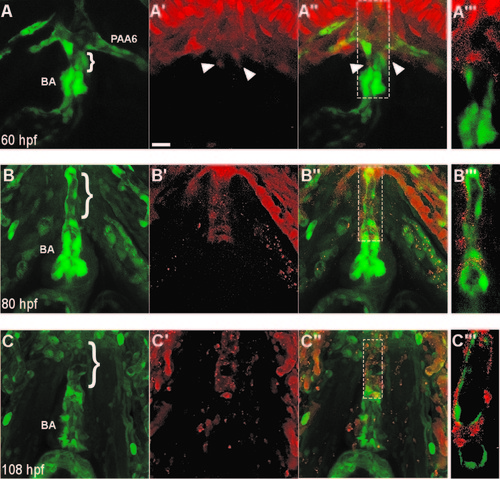
Neural crest-derived cells surround the endothelium of the ventral aorta and bulbus arteriosus. (A–A′′) A confocal projection of a ventral view of the BA and VA of a NC:NfsB-mCherry; kdrl:GFP embryo at 60 hpf (anterior toward top). Arrowheads point to neural crest cells migrating onto the VA from the 6th aortic arch artery. (B–C3) Confocal projections of BA and VA of a NC:mCherry; kdrl:GFP embryo at 80 hpf (B–B′′′ and 108 hpf (C–C′′′) show the progression of mCherry+ cells migrating along the VA. A′′′, B′′′ and C′′′, Single z section of area boxed in A′′, B′′ and C′′, respectively. Brackets mark the ventral aorta (VA); BA, bulbus arteriosis; PAA6, pharyngeal arch artery 6, scale bar=20 µm. |
|
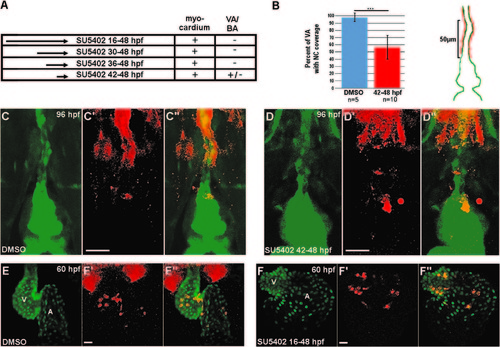
Requirement for FGF signaling in neural crest contribution to the heart. (A) Experimental design and results for FGF inhibition using SU5402. NC contribution to cardiac chambers was not affected by FGF inhibition at any time point, while NC contribution to the VA and BA was reduced by FGF inhibition. +, mCherry cells present; -, mCherry cells absent; +/-, drastically reduced mCherry cells. (B) Percent coverage of the ventral aorta by NC-derived cells for DMSO-treated control embryos, and embryos treated with SU5402 from 42 to 48 hpf. Cartoon illustrates the 50 µm area of the VA assessed for coverage by mCherry positive cells. Red represents mCherry cells and green represents the endothelium of the VA and BA. (C–D′′) Confocal projections of the BA and VA in DMSO-treated control (C–C′′) and SU5402-treated NC:mCherry; kdrl:GFP embryos (D–D′′). (E–F′′) Confocal projections of DMSO-treated control (E–E′′) and SU5402-treated NC: mCherry; myl7:GFP embryos. VA, ventral aorta; BA, bulbus arteriosus, A, atrium; V, ventricle. Scale bars=20 µm. ***p<0.001. |
|
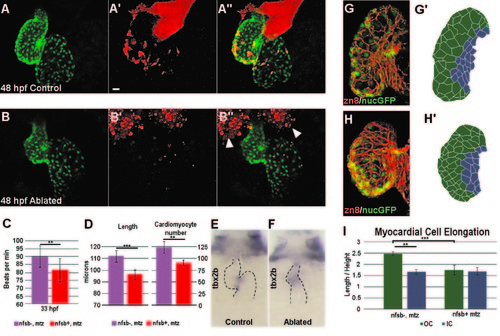
Neural crest ablation affects cardiac function and morphology. (A–B′′) Confocal projection of hearts from untreated control (A–A′′) and Mtz-treated (B–B′′) NC:NfsB-mCherry; myl7:nucGFP embryos. Note that Mtz-treated embryos had smaller ventricles. Arrowheads point to dying NCCs in surrounding tissues. Scale bar=20 µm. (C) Graph indicates heart rate in Mtz-treated NfsB-mCherry (–) controls and Mtz-treated NfsB-mCherry (+) NC-ablated embryos at 33 hpf. (D) Graph indicates ventricle length of Mtz-treated NfsB-mCherry () controls and Mtz-treated NfsB-mCherry (+) NC-ablated embryos at 48 hpf and ventricular myocardial cell number at 72 hpf. (E, F) In situ hybridization for tbx2b at 60 hpf in control (E) and NC-ablated embryos (F). Note that tbx2b expression domain is expanded in NC-ablated embryos. (G, H) Confocal projections of 80 hpf ventricles immunostained with membrane marker Zn8 from control (G) and NC-ablated embryos (H). (G2, H2) Traces of cell shape from the images shown in G and H with outer curvature cells in green and inner curvature cells in blue. (I) Quantification of the ratio between the longest and shortest axis of each outer (green) and inner (blue) curvature myocardial cell in control and NC-ablated embryos. **p<0.05; ***p<0.001. |
|
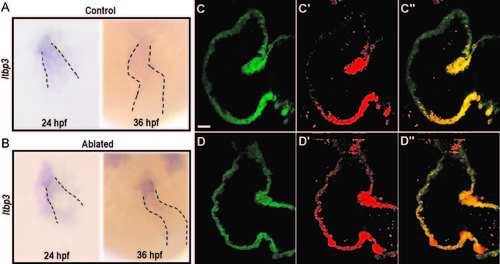
Requirement for neural crest cells in the recruitment of second heart field progenitors. (A and B) In situ hybridization for ltbp3 in control (A) and NC-ablated embryos (B). Ltbp3 expression is observed in both control and NC-ablated embryos at 24 hpf. By 36 hpf, ltbp3 expression is down-regulated in control, but is persistent in NC-ablated embryos. Dashed lines indicate location of heart. (C–D′′) Single z sections of ventricles from Mtz-treated NfsB-mCherry (-); myl7:kaede control embryo (C–C′′) and Mtz-treated NfsB-mCherry (+); myl7:kaede NC-ablated embryos (D–D′′) at 60 hpf. Kaede protein photoconverted at 26 hpf are fluorescent in red whereas newly produced protein are in green. Note that ventricular cardiomyocytes at the arterial end express green Kaede whereas red Kaede proteins are present throughout the myocardium in NC-ablated embryos, indicating a defect in the recruitment of second heart field progenitors. Scale bar=20 µm. |
|
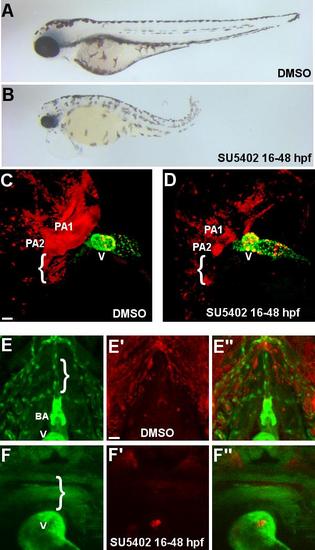
(A-B) Wide field image of embryo treated with DMSO (A) and SU5402 (B) from 16-48 hpf. (C-D) Confocal projection of a lateral view of the anterior region of NC:NfsB-mCherry; myl7:nucGFP embryo treated with DMSO (C) or SU5402 (D) from 16-48 hpf (anterior toward top). Inhibition of FGF signaling by SU5402 treatment resulted in a small ventricle and diminished NC contribution in the pharynx PA1 and PA2. Brackets indicate more posterior PAs. Scale bar=40 µm. (E-F′′) Confocal projection of the ventral view of NC:mCherry; kdrl:GFP embryos at 72 hpf. Scale bar=20 µm. (E-E′′) In DMSO-treated control embryos, the VA and BA are clearly defined and have normal contribution from mCherry positive cells. (F-F′′) SU5402 treatment perturbed VA and BA formation (note absence of kdrl:GFP) and abolished the contribution of mCherry positive in the region. The weak GFP signals in cells of non-sox10 lineages are from the ubi:switch transgene of NC:mCherry. Brackets indicate the ventral aorta. BA, bulbus arteriosus; V, ventricle. |
Reprinted from Developmental Biology, 404(2), Cavanaugh, A.M., Huang, J., Chen, J.N., Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart, 103-12, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.