- Title
-
Fgf22 regulated by Fgf3/Fgf8 signaling is required for zebrafish midbrain development
- Authors
- Miyake, A., and Itoh, N.
- Source
- Full text @ Biol. Open
|
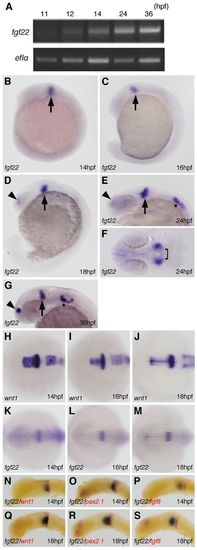
Expression of fgf22 in zebrafish embryos. (A) Amplification of fgf22 by RT-PCR at the indicated stages. The lower panel shows results for ef1α as a control. (B–S) Expression pattern of fgf22 (B–G,K–S) and wnt1 (H–J) in zebrafish embryos at the indicated stages detected by whole-mount in situ hybridization. Embryos were double-labeled for wnt1 (red) (N,Q), pax2.1 (red) (O,R), or fgf8 (red) (P,S). Lateral views with anterior to the left and dorsal to the top (B–D,G,N–S). Dorsal views with anterior to the top (F,H–M). Arrowheads and asterisks indicate the telencephalon and otic vesicles, respectively.EXPRESSION / LABELING:
|
|
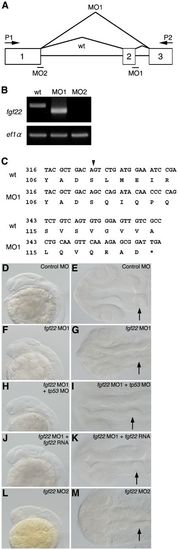
Inhibition of fgf22 functions in zebrafish embryos. (A) The coding region of fgf22 is divided by two introns. Open boxes and black lines indicate exons and introns, respectively. MO indicates the target position of fgf22 MO. (B) fgf22 cDNA was amplified from wild-type or fgf22 MO-injected embryonic cDNA by RT-PCR using P1 and P2 primers, the positions of which are indicated by arrows (A). ef1α cDNA was also amplified as a control. (C) The nucleotide sequences of fgf22 cDNAs described above were determined. Numbers for the nucleotide sequence of the coding region and the amino acid sequence are shown. Arrowheads indicate splice-sites between exons one and two. (D–M) Lateral views (D,F,H,J,L) and dorsal views (E,G,I,K,M) of control MO-injected (D,E), fgf22 MO1-injected (F,G), fgf22 MO1- and tp53 MO-injected (H,I), fgf22 MO1- and fgf22 RNA-injected (J,K), and fgf22 MO2-injected (L,M) embryos at 24 hpf are shown. PHENOTYPE:
|
|
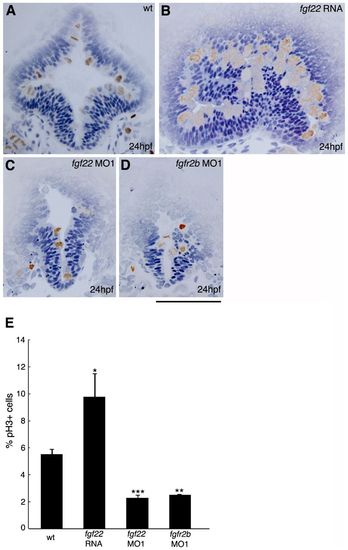
Comparison of cell proliferation in the midbrain of embryos injected with fgf22 RNA, fgf22 MO1, or fgfr2b MO1. (A–D) Wild-type embryos (A) and embryos injected with fgf22 RNA (B), fgf22 MO1 (C), or fgfr2b MO1 (D) were stained using an anti-pH3 antibody. Panels show representative transverse sections of the midbrain at 24 hpf. Scale bar: 100μm. (E) The percentage of proliferating cells labelled with anti-pH3 antibody in the midbrain of wild-type embryos and embryos injected with fgf22 RNA, fgf22 MO1, or fgfr2b MO1. Results are the mean ± S.D. for three independent sections from three embryos. The statistical significance of differences in mean values was assessed with the Student′s t-test. Asterisks indicate statistical significance compared with the wild type (*P<0.05; **P<0.01; ***P<0.001). PHENOTYPE:
|
|
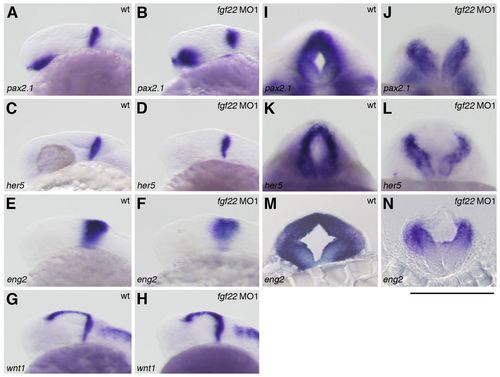
Gene expression in the MHB of the fgf22 morphants. (A–N) The expression of pax2.1 (A,B,I,J), her5 (C,D,K,L), eng2 (E,F,M,N), and wnt1 (G,H) in wild-type embryos (A,C,E,G,I,K,M) and fgf22 morphants (B,D,F,H,J,L,N) at 24 hpf. A–H are lateral views, anterior to the left; I–L are optical cross-sections of the MHB; M,N are midbrain transverse sections. (I–N) Scale bar: 100 μm. |
|
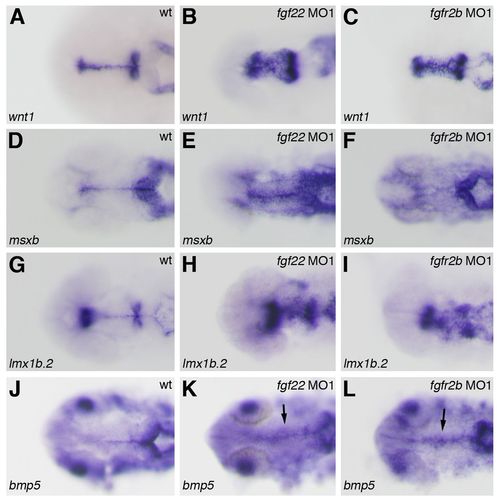
Gene expression in the midbrain roof plate of the fgf22 and fgfr2b morphants. The expression of wnt1 (A–C), msxb (D–F), lmx1b.2 (G–I), and bmp5 (J–L) in wild-type embryos (A,D,G,J) and fgf22 (B,E,H,K) and fgfr2b (C,F,I,L) morphants at 24 hpf. |
|
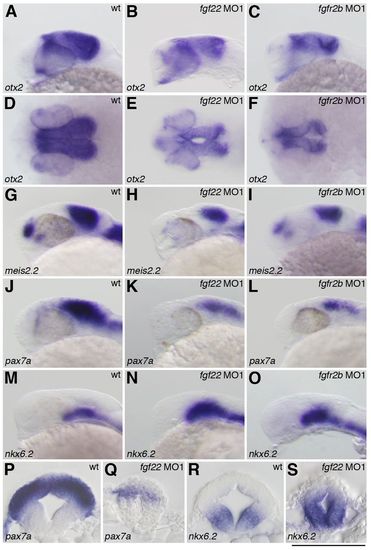
Gene expression in the midbrain of the fgf22 and fgfr2b morphants. The expression of otx2 (A–F), meis2.2 (G–I), pax7a (J–L,P,Q) and nkx6.2 (M–O,R,S) in wild-type embryos (A,D,G,J,M,P,R) and fgf22 (B,E,H,K,N,Q,S) and fgfr2b (C,F,I,L,O) morphants at 24 hpf. A–C,G–O are lateral views, anterior to the left; D–F are dorsal views; P–S are midbrain transverse sections. (P–S) Scale bar: 100 μm. |
|
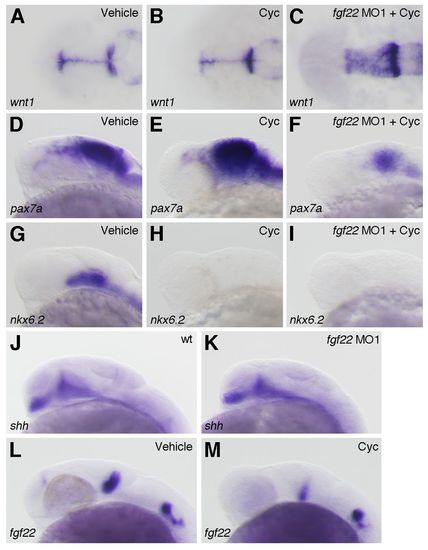
Interactions between fgf22 and Hh signaling in the midbrain. (A–I) The expression of wnt1 (A–C), pax7a (D–F) and nkx6.2 (G–I) at 24 hpf in wild-type embryos treated with 0.95% ethanol (A,D,G) or cyclopamine (B,E,H) and fgf22 morphants treated with cyclopamine (C,F,I). (J–M) The expression of shh (J,K) and fgf22 (L,M) at 24 hpf in wild-type embryos (J), fgf22 morphants (K), and wild-type embryos treated with 0.95% ethanol (L) or cyclopamine (M). EXPRESSION / LABELING:
|
|
Interactions between fgf3, fgf8 and fgf22. (A–D) The expression of fgf22 at 24 hpf in embryos injected with control MO (A), fgf3 MO (B), fgf8 MO (C), and fgf3 MO and fgf8 MO (D). (E–L) The expression of fgf3 (E–H) and fgf8 (I–L) at 24 hpf in embryos injected with control MO (E,F,I,J) and fgf22 MO (G,H,K,L). A–D,E,G,I,K are lateral views, anterior to the left; F,H,J,L are optical cross-sections of the MHB. EXPRESSION / LABELING:
|
|
Rescue of midbrain region-specific marker loss in fgf3/fgf8 morphants by fgf22 RNA. The expression of wnt1 (A,B), pax7a (C,D), and nkx6.2 (E,F) at 24 hpf in fgf3/fgf8 morphants (A,C,E) and fgf22 RNA-injected fgf3/fgf8 morphants (B,D,F). EXPRESSION / LABELING:
|
|
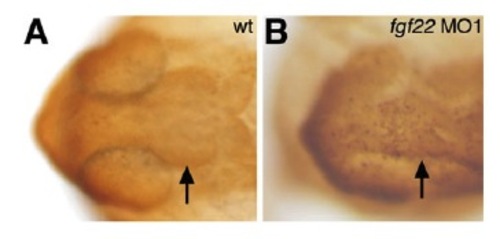
Apoptosis in the midbrain of fgf22 morphants. At 24 hpf, apoptotic cells in the midbrain of the wild-type (A) and fgf22 MO1-injected (B) embryos were marked via TUNEL. Dorsal view with anterior to the left. |
|
Inhibition of fgf22 and fgfr2b functions in zebrafish embryos. (A) The cDNA of fgfr2b and fgfr2c was amplified from wild-type or Fgfr2b MO-injected embryonic cDNA by RT-PCR using forward and reverse primers. ef1a cDNA was also amplified as a control. (B–G) The expression of wnt1 (B,C), pax7a (D,E), and nkx6.2 (F,G) at 24 hpf in fgf22 MO2-injected (B,D,F), and fgfr2b MO2-injected (C,E,G) embryos. |
|
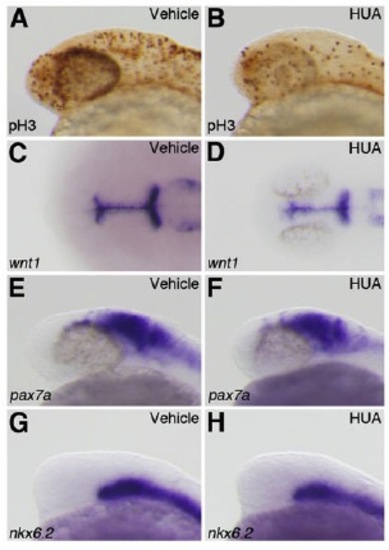
Cellular proliferation does not contribute to the reduction of dorsal midbrain. (A,B) Wild-type embryos treated with 4% DMSO (A) or HUA (B) were stained using an anti-pH3 antibody. (C–H) The expression of wnt1 (C,D), pax7a (E,F), and nkx6.2 (G,H) at 24 hpf in wild-type embryos treated with 4% DMSO (C,E,G) or HUA (D,F,H). |