- Title
-
Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar)
- Authors
- Reifers, F., Walsh, E.C., Léger, S., Stainier, D.Y.R., and Brand, M.
- Source
- Full text @ Development
|
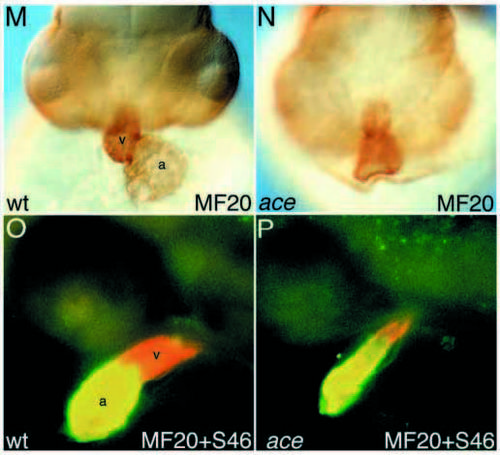
Circulation, blood vessel formation and abnormal heart morphology in acerebellar mutants. (A,B) Overview of blood vessel system in a day-2 wild-type (A) and an ace (B) larva. (C,D) Confocal close-up of the blood vessels in the head in a wildtype (C) and an ace (D) larva on day 2, with the main vessels in the tectum, at the midhindbrain boundary and in the hindbrain being affected in ace mutants (arrows). The dashed circle in D marks the position of the eye. (E,F) The vessel at the mid-hindbrain boundary (E, arrowhead) is missing in ace mutant embryos (F, asterisk) at 24 hpf, detected by in situ hybridization with flk-1. aa, aortic arches; acv, anterior cardinal vein; h, heart; hbv, hindbrain vessel; mhbv, mid-hindbrain vessel; tv, tectal vessel; sv, segmental vessel. (G-J) Malformation of the heart in acerebellar larva (I) compared to wild type (G). G and I are lateral views of living larvae, H and J are schematic representation of the main structures in G and I. (K,L) Endocardium and myocardium are present and appear normal in ace mutants. (M,N) The heart is malformed and shortened in ace mutants, as shown by MF20 antibody staining (frontal view). MF20 antibody reacts with both cardiac chambers, whereas S46 specifically stains the atrium. (O,P) Double staining with MF20 and S46 facilitated measuring the chambers and the average length of ace hearts (83±16%, n=19) was found to be similar to the wild-type hearts (100±8%, n=10), whereas the ventricle was reduced in ace mutant embryos. In wild-type embryos, the ventricle contributes 39±4% (n=10) to the total heart length at 26 hpf, compared to only 24±7% (n=19) in ace mutants. Ventricle reduction becomes more pronounced, but is still variable at later stages: at 33 hpf, the ventricle is severely reduced or absent in 60% of all ace embryos (n=55), and less affected in the remaining embryos. (G-N) Day-2 larvae, (O,P) 26 hpf. a, atrium; bc, blood cells; e, endocard; h, heart; l, lens; m, myocard; p, pericard; v, ventricle; y, yolk. PHENOTYPE:
|
|
Cardiac expression of fgf8 in relation to heart marker genes in wild-type embryos. (A-F) fgf8 expression at various stages of development, as indicated. B and D are cross sections of A and C, respectively. (A,B) Cells in lateral plate mesoderm express fgf8 (arrows) at the 3-somite stage. (C,D) Expression bilaterally to the neural tube including cardiogenic fields (arrowheads in C and arrow in D) at the 8-somite stage. (E) Ring-shaped expression in the heart at 19-somite stage. (F) Expression is predominantly in the ventricle at 36 hpf. (F) Dissected heart. (A) Anterior to the top; (C,E) anterior to the left; a, atrium; h, heart; mhb, mid-hindbrain boundary; os, optic stalks; r2, rhombomere 2; r4, rhombomere 4; v, ventricle. (G-U) Double in situ hybridization with fgf8 (black) and indicated heart markers (red fluorescence) of wild-type embryo at given stages. (G-I) fgf8 is expressed in close proximity to nkx2.7. (J-L) fgf8 is expressed close to gata4. (M-O) fgf8 expression partially overlaps nkx2.5 expression (star marks the same cell in M and N). (P-R) fgf8 expression partially overlaps gata4 expression. (S-U) fgf8 expression strongly overlaps nkx2.5 expression. Brackets indicate the two expression domains. (V,W) Summary of fgf8 expression relative to the described heart marker genes at given stages (grey, fgf8; red, nkx2.5; blue, gata4, black, triple overlap). Embryos in G,H,J,K,M,N,P,Q,S,T are flat mounted, anterior to the left; G,J,M,P,S are bright-field images, H,K,N,Q,T are fluorescent images of the same embryos; I,L,O,R,U are cross sections at the indicated levels, lateral is to the right. mhb, mid-hindbrain boundary; r4, rhombomere 4. EXPRESSION / LABELING:
|
|
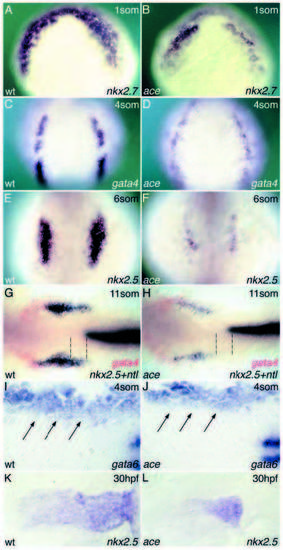
Fgf8 is required for cardiac marker gene expression. Stages and markers as indicated. (A,B) Expression of nkx2.7 in wild-type (A) and ace (B) embryos. (C,D) Expression of gata4 in wild-type (C) and ace (D) embryos. (E-H) Expression of nkx2.5 in wild-type (E,G) and ace (F,H) embryos. Notice the differential sensitivity of the posterior nkx2.5 domain (dashed lines). (I,J) Expression of gata6 (arrows) in wild-type (I) and ace (J) embryos. (K,L) Late expression of nkx2.5 in dissected hearts of wild-type (K) and ace (L) embryos. A-F show dorsal views of whole embryos, anterior to the top. Embryos in G-J are flat mounted, anterior to the left. Embryos in G,H are stained for nkx2.5 and ntl (black) and gata4 (red). Embryos in I and J are double-stained for myoD to genotype the embryos (Reifers et al., 1998). EXPRESSION / LABELING:
|
|
Fgf8 functions in the heart primordium. (A,B) Misexpression of fgf8 in ace embryos by RNA injection. Localization of lacZ co-injected cells with an antibody to β-gal (brown). fgf8 RNA injection can restore gata4 (A), but not nkx2.5 (B) expression in mutant embryos. (C,D) Expression of XFD in wild-type embryos by RNA injection. Localization of lacZ co-injected cells with an antibody to β-gal. XFD RNA injection suppresses gata4 (C) and nkx2.5 (D) expression in wild-type embryos. (E,F) Implantation of a Fgf8-soaked bead (arrow) after gastrulation can rescue nkx2.5 expression in ace embryos (E), while PBS bead gives no effect (F). (G) Implantation of a Fgf8-soaked bead (arrow) after gastrulation can caudally extend (dashed line) the endogenous nkx2.5 expression domain in wild-type embryos. (H) Model of Fgf8 function in cardiogenesis. All embryos are oriented with anterior to the left; A,B,E-G are flat mounted; C,D are dorsal views of whole embryos. nkx2.5 and gata4 were detected by in situ hybridization. (I-P) Fgfr inhibitor treatments. (I-K) 10-somite stage, (L,M) 15-somite stage, (N) 18- somite stage, (O,P) 26 hpf. (I-N) nkx2.5 and myoD expression detected by ISH; dorsal view of whole embryo, anterior to the left. (O,P) Double staining with MF20 and S46 antibodies, lateral view, anterior to the left. (I-N) Embryos have been treated with SU5402 from the 1-somite stage onwards for 2 hours (J,M) or continuously (K,N). (I,L) Non-treated control embryos. nkx2.5 expression is absent after short exposure to the inhibitor at the 10-somite stage (J), but begins to recover at the 15-somite stage (M). Continuous treatment results in permanent loss of nkx2.5 expression (K,N). (O,P) Embryos that have been continuously treated with SU5402 from the 18-somite stage onwards show no ventricular tissue (P); compare with the untreated control (O). Arrow marks the ventricle, arrowheads point to the MF20-positive somites. a, atrium; v, ventricle. |