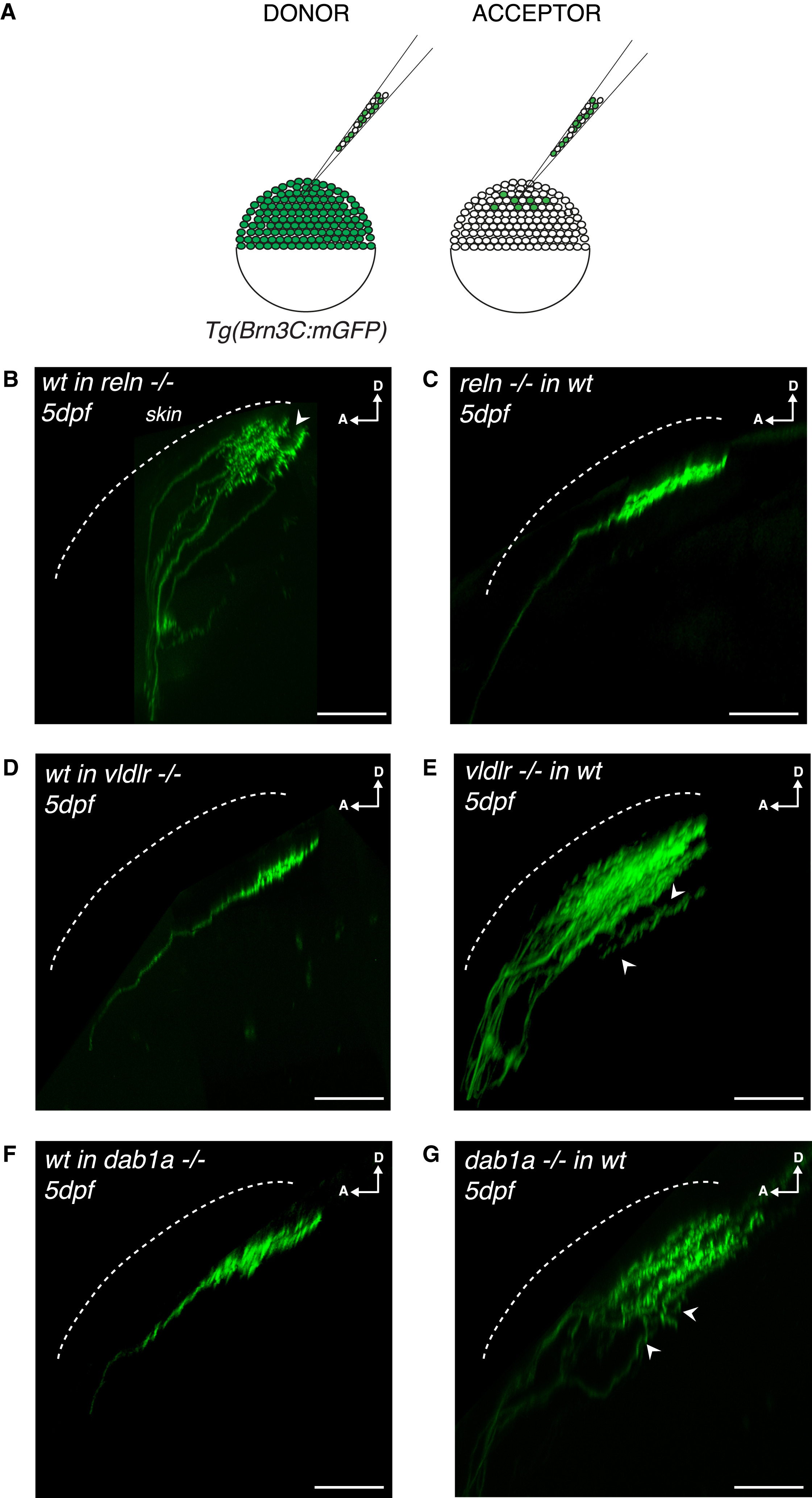
Fig. 3
Tectum-Derived Reelin and RGC-Derived VLDLR/Dab1a Regulate the Establishment of RGC Synaptic Laminae in the Tectal Neuropil
(A) Schematics of blastula transplants from RGC-specific Tg(Brn3c:mGFP) donors supplying acceptor embryos of varying genotypes with green-labeled RGCs of donor genotype.
(B) Side view of RGC axons at 5 dpf after blastula stage transplantations from a wild-type donor into a reelin mutant acceptor. Aberrant laminations (white arrowhead) were detected in all larvae analyzed (n = 8/8).
(C) Side view of a RGC axon in the tectal neuropil at 5 dpf after blastula stage cell transplantation from a reelin mutant donor into a wild-type acceptor. No synaptic lamination defects were detected in all larvae analyzed (n = 0/12), suggesting that tectum-derived Reelin and not Reelin expressed by RGCs controls single synaptic lamina targeting.
(D) Side view of a RGC axon in the tectal neuropil at 5 dpf after blastula stage cell transplantation from a wild-type donor into a vldlr−/− acceptor. No synaptic lamination defects were observed in analyzed larvae (n = 0/6).
(E) Side view of RGC axons in the tectal neuropil at 5 dpf after blastula stage cell transplantation from a vldlr−/− mutant donor into a wild-type acceptor. Aberrant laminations (white arrowhead) were detected in most larvae (n = 5/10), suggesting that vldlr expressed by RGCs influences single lamina targeting by RGCs.
In (A)–(E), scale bars, 30 μm. D, dorsal; A, anterior.
(F) Side view of a RGC axon in the tectal neuropil at 5 dpf after blastula stage cell transplantation from a wild-type donor into a dab1a−/− acceptor. No synaptic lamination defects were observed in analyzed larvae (n = 0/7).
(G) Side view of RGC axons in the tectal neuropil at 5 dpf after blastula stage cell transplantation from a dab1a−/− mutant donor into a wild-type acceptor. Aberrant laminations (white arrowheads) were detected in most larvae (n = 5/9), suggesting that dab1a expressed by RGCs is involved in single lamina targeting by RGCs.