|
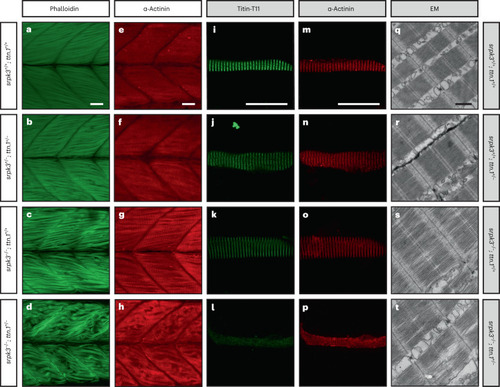
ttn.1 heterozygosity induces a severe phenotype in homozygous srpk3-null mutant zebrafish larvae. a–h, Lateral view of Alexa Fluor phalloidin filamentous actin (green) and α-actinin Z-band marker (red) staining in skeletal fast muscle fibers in WT (a,e), srpk3+/−; ttn.1+/− (b,f), srpk3−/−; ttn.1+/+ (c,g) and srpk3−/−; ttn.1+/− larvae (d,h) at 5 dpf. Compared to WT (a,e) or double heterozygotes (srpk3+/−; ttn.1+/−; b,f), homozygous srpk3-null alone only causes very mild muscle fiber defects (c,g), while ttn.1 heterozygosity in homozygous srpk3−/− larvae severely affects muscle fiber integrity (d,h). i–t, Isolated myofiber immunostaining and electron microscopy (EM) in skeletal fast muscle fibers in WT (i,m,q), srpk3+/+; ttn.1+/− (j,n,r), srpk3−/−; ttn.1+/+ (k,o,s) and srpk3−/−; ttn.1+/− (l,p,t) larvae at 5 dpf. Isolated myofiber immunostaining showed that titin expression is largely reduced in the double mutant (srpk3−/−; ttn.1+/−; l,p) but not in the single heterozygous ttn.1 mutant (srpk3+/+; ttn.1+/−; j,n) or the srpk3-null (srpk3−/−; ttn.1+/+; k,o). EM showed that srpk3-null zebrafish (srpk3−/−; ttn.1+/+; s) had well-defined sarcomeres, with mildly disorganized myofibrils. The double-mutant fish (srpk3−/−; ttn.1+/−; t) displayed pronounced disruption of the sarcomere structure. White scale bars are 25 µm. Black scale bar is 500 nm. Representative images from >15 pooled fish per genotype.
|