Fig. 6
- ID
- ZDB-FIG-160714-21
- Publication
- Richardson et al., 2016 - Re-epithelialization of cutaneous wounds in adult zebrafish uses a combination of mechanisms at play during wound closure in embryonic and adult mammals
- Other Figures
- All Figure Page
- Back to All Figure Page
|
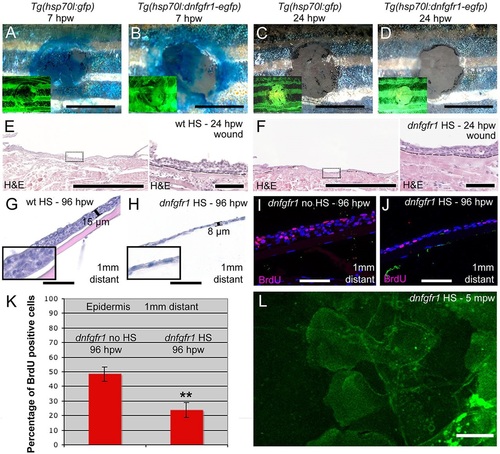
FGF signalling is dispensable for re-epithelialization, but is required for keratinocyte proliferation and epidermal regrowth. (A-D) Methylene Blue penetration assay at 7hpw (A,B) and 24hpw (C,D) revealing unaltered re-epithelialization rates of full-thickness wounds between heat-shocked control Tg(hsp70l:gfp) (A,C) and Tg(hsp70l:dnfgfr1-egfp) (B,D). Insets show fluorescence of transgene-encoded GFP or dnFGFR-GFP fusion proteins, indicating strong transgene expression. (E,F) Histological analysis of wound epidermis at 24hpw revealing unaltered thicknesses of neo-epidermis between heat-shocked wild-type control (E) and Tg(hsp70l:dnfgfr1-egfp) fish (F). Right panels show magnified views of regions boxed in left panels. (G,H) At 96hpw, the epidermis 1mm distant from the wound of the heat-shocked Tg(hsp70l:dnfgfr1-egfp) fish (H) is much thinner than in the heat-shocked non-transgenic control (G). Insets show magnified views. (I,J) BrdU incorporation revealing significantly reduced epidermal proliferation in heat-shocked (J) compared with non-heat shocked Tg(hsp70l:dnfgfr1-egfp) fish (I) 1mm distant from the wound. (K) Quantification of BrdU incorporation rates (percentage of cells) from images as in I,J. (L) Live image of LE of heat-shocked Tg(hsp70l:dnfgfr1-egfp) fish after partial-thickness injury, revealing normal protrusive activity (see Fig. 4E as control). Scale bars: 2mm in A-D; 500µm in left panels of E,F; 50µm in G-J and right panels of E,F; 10µm in L. |
| Gene: | |
|---|---|
| Fish: | |
| Condition: | |
| Anatomical Term: | |
| Stage: | Adult |