Fig. 5
- ID
- ZDB-FIG-130409-26
- Publication
- Guner-Ataman et al., 2013 - Zebrafish second heart field development relies on progenitor specification in anterior lateral plate mesoderm and nkx2.5 function
- Other Figures
- All Figure Page
- Back to All Figure Page
|
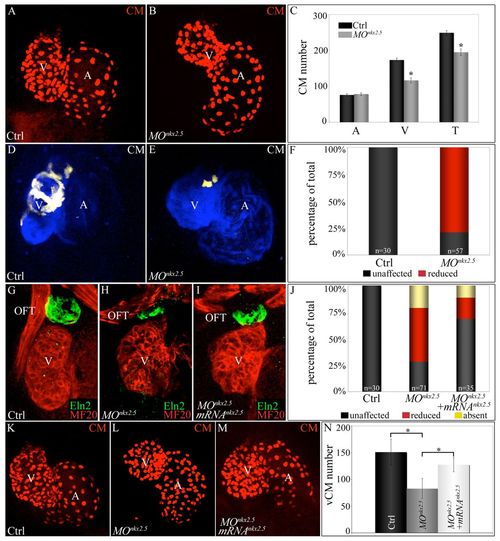
Morpholino-mediated inhibition of nkx2.5 pre-mRNA splicing elicits SHF phenotypes. (A-C) nkx2.5 morphants display ventricular cardiomyocyte deficits. z-stack images of hearts in control (A) and nkx2.5 morpholino-injected (B) Tg(cmlc2:dsRed2-nuc) embryos imaged at 72 hpf. (C) Average numbers of atrial (A), ventricular (V) and total (T) cardiomyocytes in control (n=6) and morphant (n=11) embryos. Error bars represent one s.d. *P<0.001. (D-F) SHF-derived ventricular myocardium is specifically reduced in nkx2.5 morphants. Control (D) and morpholino-injected (E) Tg(ltbp3:TagRFP2Acre); Tg(cmlc2:CSY) embryos imaged in the blue and yellow channels (merged images shown) by confocal microscopy at 72 hpf. (F) The percentages of embryos with reduced ventricular ZsYellow expression. The n values are reported in the graph. (G-J) nkx2.5 morphants exhibit reductions in Eln2+ OFT cells, a phenotype partially rescued by co-injection of nkx2.5 mRNA. Control (G; unaffected), morphant (H; reduced) and mRNA-injected morphant (I; unaffected) embryos were co-stained at 72 hpf with MF20 and anti-Eln2 antibodies to label myocardium (red) and OFT smooth muscle (green), respectively. (J) Percentages of embryos with unaffected, reduced or absent Eln2 staining. The n values are reported in the graph. (K-N) Injection of nkx2.5 mRNA partially rescues the ventricular cardiomyocyte deficit in nkx2.5 morphants. Control (K), morphant (L) and mRNA-injected morphant (M) Tg(cmlc2:dsRed2-nuc) embryos imaged by confocal microscopy at 72 hpf. (N) Average numbers of ventricular cardiomyocytes (vCMs) in each experimental group (n=4 per cohort). Error bars represent one s.d. *P<0.05. CM, cardiomyocytes; V, ventricle; A, atrium; OFT, outflow tract; Ctrl, control; MO, morpholino. |