- Title
-
Mis-localization of endogenous TDP-43 leads to ALS-like early-stage metabolic dysfunction and progressive motor deficits
- Authors
- Hu, Y., Hruscha, A., Pan, C., Schifferer, M., Schmidt, M.K., Nuscher, B., Giera, M., Kostidis, S., Burhan, Ö., van Bebber, F., Edbauer, D., Arzberger, T., Haass, C., Schmid, B.
- Source
- Full text @ Mol. Neurodegener.
|
Biochemical characterization of ΔNLS-Tardbp fish. |
|
Morphological characterization of CytoTDP fish. |
|
Tardbp localization in CytoTDP fish. |
|
Age dependent movement phenotypes. |
|
Reduction of motor neurons and NMJ degeneration in CytoTDP. |
|
Mislocalization of endogenous TDP-43 causes muscle atrophy. |
|
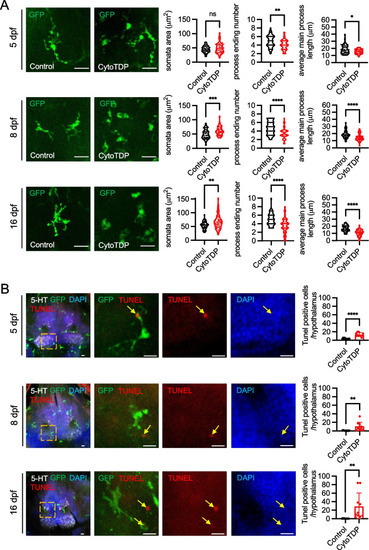
Microglia proliferation in the hypothalamus of CytoTDP. |
|
Microglia activation in the hypothalamus of CytoTDP. |
|
CytoTDP affects key metabolic processes. |