- Title
-
The physical and cellular mechanism of structural color change in zebrafish
- Authors
- Gur, D., Moore, A.S., Deis, R., Song, P., Wu, X., Pinkas, I., Deo, C., Iyer, N., Hess, H.F., Hammer, J.A., Lippincott-Schwartz, J.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
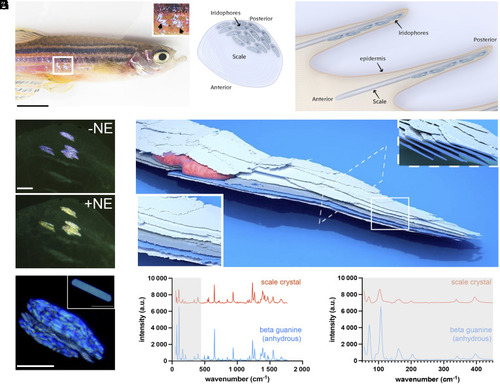
Anatomy, crystal properties, and color change capabilities of the zebrafish scale iridophores. ( |
|
NE-induced changes in crystal tilt, crystal spacing, and cellular optical properties. ( |
|
Color change in scale iridophores is driven by the tilting of crystals. ( |
|
Microtubules and the microtubule minus end-directed motor protein dynein drive NE-induced color change. ( |