- Title
-
Sleep pressure modulates single-neuron synapse number in zebrafish
- Authors
- Suppermpool, A., Lyons, D.G., Broom, E., Rihel, J.
- Source
- Full text @ Nature
|
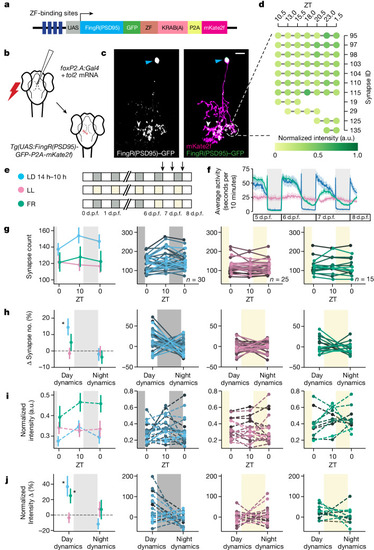
Single-neuron synapse tracking across day–night cycles reveals diverse dynamics. |
|
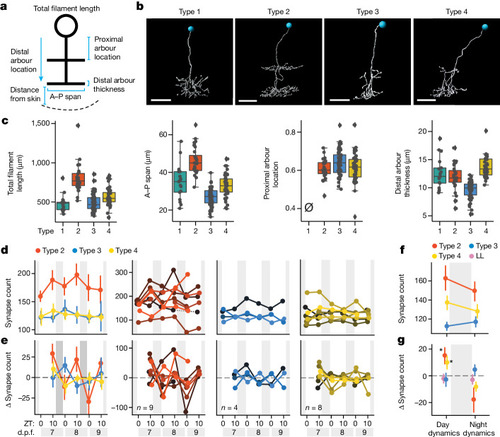
Subtype-specific synapse changes in FoxP2.A tectal neurons over 3 days. |
|
Synapse counts of neurons are modulated by sleep and SD. |
|
Single-neuron synapse loss during sleep is driven by boosting adenosine and blocking noradrenaline. |