- Title
-
Dual modifying of MAVS at lysine 7 by SIRT3-catalyzed deacetylation and SIRT5-catalyzed desuccinylation orchestrates antiviral innate immunity
- Authors
- Liu, X., Zhu, C., Jia, S., Deng, H., Tang, J., Sun, X., Zeng, X., Chen, X., Wang, Z., Liu, W., Liao, Q., Zha, H., Cai, X., Xiao, W.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
SIRT3 activates the MAVS-mediated innate antiviral response. (A–C) qPCR analysis of IFNβ (A), CXCL10 (B), and ISG15 (C) mRNA in H1299 cells transfected with indicated plasmids for 24 h, and then left uninfected (UI) or infected with SeV for 8 h. (D–F) qPCR analysis of IFNβ (D), CXCL10 (E), and ISG15 (F) mRNA in SIRT3−/− or SIRT3+/+ H1299 cells left UI or infected with SeV for 8 h. (G) Immunoblotting (IB) of whole cell lysates using the indicated antibodies in SIRT3+/+ and SIRT3−/− H1299 cells UI or infected with SeV for 4 or 8 h. (H) Quantitation of phosphorylated IRF3 (p-IRF3) and SeV proteins shown in (G). (I) SIRT3−/− or SIRT3+/+ H1299 cells were infected with VSV-GFP virus (MOI = 0.1) for 12 h, and viral infectivity was detected by fluorescence microscopy and immunoblotting. (J)Schematic of the working model of high molecular weight poly I:C (poly I:C-HMW) and low molecular weight poly I:C (poly I:C-LMW). (K) qPCR analysis of IFNβ mRNA in H1299 cells transfected with the indicated plasmids for 24 h, and then transfected without (UT) or with poly I:C HMW or poly I:C LMW for 8 h. (L) qPCR analysis of IFNβ mRNA in SIRT3−/− or SIRT3+/+ H1299 cells transfected without (UT) or with high molecular weight poly I:C (poly I:C HMW) or low molecular weight poly I:C (poly I:C LMW) for 8 h. (M) ISRE reporter activity by cotransfection of HA-MAVS together with Flag empty vector (Flag empty) or with Flag-SIRT3 in HEK293T cells for 24 h. (N) IFNβ promoter activity by cotransfection of HA-MAVS together with Flag empty vector (Flag empty) or with Flag-SIRT3 in HEK293T cells for 24 h. (O) qPCR analysis of IFNβ mRNA upon cotransfection of HA-MAVS together with Flag empty vector (Flag empty) or with Flag-SIRT3 in HEK293T cells for 24 h. (P–S) qPCR analysis of Ifnβ (P), Isg15 (Q), Cxcl10 (R), and Ifit1 (S) mRNA in Mavs+/+ and Mavs−/− MEF cells stably expressing empty vector or Sirt3 (lentivirus infection), followed by infection without (UI) or with VSV for 8 h. (T and U) Mavs+/+ and Mavs−/− MEF cells stably expressing empty vector or Sirt3 were infected with VSV-GFP virus (MOI = 0.1) for 12 h, and viral infectivity was detected by fluorescence microscopy (T) and IB (U). (V–X) qPCR analysis of Ifnβ (V), Isg15 (W), and Cxcl10 (X) mRNA in Mavs+/+ and Mavs−/− MEF cells stably expressing empty vector or Sirt3, followed by transfection without (UT) or with high molecular weight poly I:C (poly I:C HMW) or low molecular weight poly I:C (poly I:C LMW) for 8 h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data from at least three independent experiments (mean ± SD) (A–F, K–S, and V–X]. |
|
SIRT3 interacts with MAVS to enhance MAVS activation depending on its enzymatic activity. (A and B) Co-IP of Flag-SIRT3 with HA-MAVS and vice versa. HEK293T cells were cotransfected with the indicated plasmids for 24 h. Anti-HA (A) or anti-Flag (B) antibody-conjugated agarose beads were used for IP, and the interaction was detected by immunoblotting with the indicated antibodies. (C) Endogenous interaction between MAVS and SIRT3. Anti-MAVS antibody was used for IP, and normal mouse IgG was used as a control. (D) Colocalization of SIRT3 and MAVS. H1299 cells were transfected with Flag-SIRT3 for 24 h, followed by UI or infected with VSV for 6 h. Confocal microscopy image of Flag-SIRT3 was detected by immunofluorescence staining with anti-Flag antibody, and endogenous MAVS was detected by immunofluorescence staining with anti-MAVS antibody, (Scale bar, 10 µm.) (E) H1299 cells were transfected with Flag-SIRT3 or Flag empty vector as control for 24 h, followed by UI (−) or infected with VSV (+) infection for 6 h. In situ PLAs of the SIRT3-MAVS interaction in H1299 cells with indicated combinations using anti-Flag and anti-MAVS antibodies. Quantification analysis of the SIRT3-MAVS interaction is shown in the Right figure. (Scale bar, 25 µm.) (F and G) qPCR analysis of IFNβ (F) and CXCL10 (G) mRNA in A549 cells stably expressing empty vector, SIRT3, or the enzymatically inactive mutant SIRT3-H248Y (lentivirus infection), followed by infection without (UI) or with VSV for 8 h. (H–J) A549 cells stably expressing empty vector, SIRT3, or the enzymatically inactive mutant SIRT3-H248Y were infected with VSV-GFP virus (MOI = 0.1) for 16 h, and viral infectivity was detected by flow cytometry analysis (n = 3) (H), fluorescence microscopy (I), or IB (J). (K and L) qPCR analysis of IFNβ (K) and CXCL10 (L) mRNA in SIRT3-deficient A549 cells (SIRT3−/−) transfected with empty vector, Myc-tagged SIRT3 or the enzymatically inactive mutant SIRT3-H248Y for 24 h, followed by UI or infected with VSV for 8 h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data from at least three independent experiments (mean ± SD) (E–H, K, and L). |
|
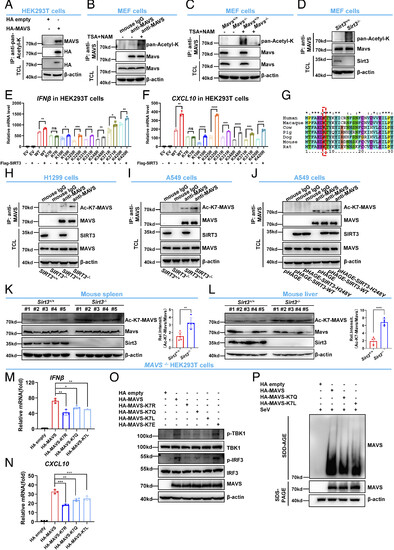
SIRT3 deacetylates MAVS at lysine 7. (A) HEK293T cells were transfected with HA empty vector (HA empty) or HA-MAVS for 24 h. Anti-pan-acetyl-K antibody was used for IP, followed by immunoblotting with anti-MAVS antibody. (B) MEF cell lysates were immunoprecipitated with anti-MAVS antibody or mouse IgG control, followed by immunoblotting with anti-pan-acetyl-K antibody. (C) Mavs−/− or Mavs+/+ MEF cells were treated with (+) or without (−) TSA and NAM for 4 h, and the cell lysates were immunoprecipitated with anti-MAVS antibody, followed by immunoblotting with anti-pan-Acetyl-K antibody. (D) Cell lysates from Sirt3−/− or Sirt3+/+ MEF cells were immunoprecipitated with anti-MAVS antibody, followed by immunoblotting with anti-pan-Acetyl-K antibody. (E and F) qPCR analysis of IFNβ (E) and CXCL10 (F) mRNA in HEK293T cells transfected with HA-MAVS WT or mutants together with (+) or without (−) Flag-SIRT3 for 24 h. EV, empty vector. (G) Sequence alignment of partial MAVS (1 to 30 amino acids) from the human, macaque, cow, pig, dog, mouse, and rat. The red box indicates the conserved lysine 7 (K7). (H) Cell lysates from SIRT3−/− or SIRT3+/+ H1299 cells were immunoprecipitated with anti-MAVS antibody or mouse IgG control, followed by immunoblotting with anti-Ac-K7-MAVS antibody. (I) Cell lysates from SIRT3−/− or SIRT3+/+ A549 cells were immunoprecipitated with anti-MAVS antibody or mouse IgG control, followed by IB with anti-Ac-K7-MAVS antibody. (J) Cell lysates from A549 cells stably expressing empty vector, SIRT3, or the enzymatically inactive mutant SIRT3-H248Y were immunoprecipitated with anti-MAVS antibody or mouse IgG control, followed by immunoblotting with anti-Ac-K7-MAVS antibody. (K and L) Disruption of Sirt3 in mice increased MAVS acetylation in mouse spleen (K) and liver (L). Proteins extracted from spleens (K) and livers (L) of Sirt3−/− or Sirt3+/+ littermates (n = 5 per group) were analyzed with the indicated antibodies. MAVS acetylation was determined using anti-Ac-K7-MAVS antibody. Relative acetylation levels were quantified (Right). (M and N) qPCR analysis of IFNβ (M) and CXCL10 (N) mRNA in MAVS−/− HEK293T cells transfected with HA-MAVS, or its acetylation-deficient mutant (HA-MAVS-K7R), or its acetylation mimic-mutant (HA-MAVS-K7Q or HA-MAVS-K7L) for 24 h. (O) IB of TBK1 and IRF3 phosphorylation in MAVS−/− HEK293T cells transfected with HA-MAVS, or its acetylation-deficient mutant (HA-MAVS-K7R), or its acetylation mimic-mutant (HA-MAVS-K7Q or HA-MAVS-K7L), or its succinylation mimic-mutant (HA-MAVS-K7E) for 24 h. (P) SDD-AGE analysis of MAVS aggregates in MAVS−/− HEK293T cells transfected with HA-MAVS or its acetylation-mimic mutant (HA-MAVS-K7Q or HA-MAVS-K7L) for 24 h, followed by infection with SeV for 12 h. SDS–PAGE immunoblotting was used as a loading control. ns, not significant (P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data from at least three independent experiments (mean ± SD) (E, F, and K–N). |
|
Sirt3-deficient mice are more susceptible to viral infection. (A) Disruption of Sirt3 in mice increased MAVS acetylation in mouse spleen upon VSV infection. Proteins extracted from spleens of Sirt3−/− and Sirt3+/+ littermates injected intraperitoneally with VSV [3 × 107 plaque-forming units (PFU) per mouse] for 24 h (n = 5 per group) were analyzed with the indicated antibodies. MAVS acetylation was detected by anti-Ac-K7-MAVS antibody and the relative acetylation level was quantified. (B) Disruption of Sirt3 in mice increased MAVS acetylation in mouse liver after VSV infection. Proteins extracted from livers of Sirt3−/− and Sirt3+/+ littermates injected intraperitoneally with VSV [3 × 107 PFU per mouse] for 24 h (n = 5 per group) were analyzed with the indicated antibodies. MAVS acetylation was detected by anti-Ac-K7-MAVS antibody and the relative acetylation level was quantified. (C–H) qPCR analysis of Ifnβ (C), Ifnα1 (D), Ifnα4 (E), Cxcl10 (F), Cxcl11 (G), and Ifit1 (H) mRNA in the spleens of Sirt3−/− and Sirt3+/+ mice injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (I–N) qPCR analysis of Ifnβ (I), Ifnα1 (J), Isg15 (K), Cxcl10 (L), Rig-I (M), and Ifit1 (N) mRNA in the lungs of Sirt3−/− and Sirt3+/+ mice injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (O–T) qPCR analysis of Ifnβ (O), Ifnα1 (P), Isg15 (Q), Cxcl10 (R), Rig-I (S), and Ifit1 (T) mRNA in the livers of Sirt3−/− and Sirt3+/+ mice injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (U) Survival (Kaplan–Meier curve) of Sirt3−/− and Sirt3+/+ mice (n = 8) injected intraperitoneally with a high dose of VSV (3 × 107 PFU per mouse) and monitored for 10 d. *P < 0.05, using the log-rank test (Mantel-Cox). (V) ELISA of Ifnβ in serum from the Sirt3−/− (n = 5) and Sirt3+/+ mice (n = 5) injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (W) Hematoxylin-and-eosin-stained (H&E) images of lung sections from mice in (V). (Scale bar, 50 μm.) (X) Schematic of the working model for two RNA viruses, VSV and EMCV. (Y and Z) qPCR analysis of Ifnβ (Y) and Cxcl10 (Z) mRNA in the spleens of Sirt3−/− and Sirt3+/+ mice injected intraperitoneally with EMCV (1 × 106 PFU per mouse) or PBS control for 24 h. ns, not significant (P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data from at least three independent experiments (mean ± SD) (A–T, V, Y, and Z). |
|
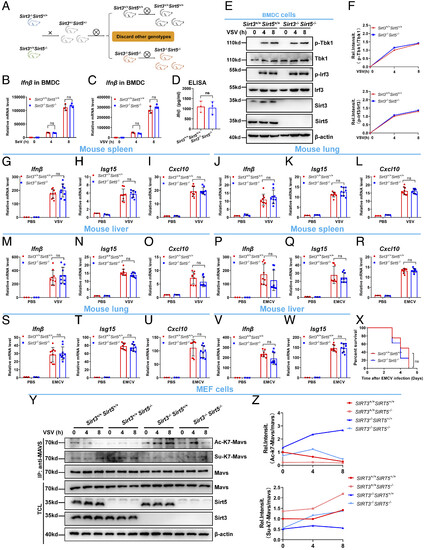
Disruption of Sirt5 in Sirt3-deficient mice counteracts the viral susceptibility exhibited in Sirt3-deficient mice. (A) Schematic of generation of Sirt3 and Sirt5 double knockout mice (Sirt3−/−Sirt5−/−). (B) qPCR analysis of Ifnβ mRNA in Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ BMDCs infected without (0) or with SeV for 4 h or 8 h. (C) qPCR analysis of Ifnβ mRNA in Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ BMDCs infected without (0) or with VSV for 4 h or 8 h. (D) ELISA of Ifnβ in supernatants of Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ BMDCs infected with VSV for 12 h. (E) IB of Tbk1 and Irf3 phosphorylation in Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ BMDCs infected without (0) or with VSV for 4 h or 8 h. (F) Quantitation of phosphorylated Tbk1 (p-Tbk1) and Irf3 (p-Irf3) proteins shown in (E). (G–I) qPCR analysis of Ifnβ (G), Isg15 (H), and Cxcl10 (I) mRNA in the spleens of Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ mice injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (J–L) qPCR analysis of Ifnβ (J), Isg15 (K), and Cxcl10 (L) mRNA in the lungs of Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ mice injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (M–O) qPCR analysis of Ifnβ (M), Isg15 (N), and Cxcl10 (O) mRNA in the livers of Sirt3−/−Sirt5−/− or Sirt3+/+Sirt5+/+ mice injected intraperitoneally with VSV (3 × 107 PFU per mouse) or PBS control for 24 h. (P–R) qPCR analysis of Ifnβ (P), Isg15 (Q), and Cxcl10 (R) mRNA in the spleens of Sirt3−/−Sirt5−/− and Sirt3+/+Sirt5+/+ mice injected intraperitoneally with EMCV (1 × 106 PFU per mouse) or PBS control for 24 h. (S–U) qPCR analysis of Ifnβ (S), Isg15 (T), and Cxcl10 (U) mRNA in the lungs of Sirt3−/−Sirt5−/− and Sirt3+/+Sirt5+/+ mice injected intraperitoneally with EMCV (1 × 106 PFU per mouse) or PBS control for 24 h. (V and W) qPCR analysis of Ifnβ (V) and Isg15 (W) mRNA in the livers of Sirt3−/−Sirt5−/− and Sirt3+/+Sirt5+/+ mice injected intraperitoneally with EMCV (1 × 106 PFU per mouse) or PBS control for 24 h. (X) Survival (Kaplan–Meier curve) of Sirt3−/−Sirt5 (n = 8) and Sirt3+/+Sirt5+/+ mice (n = 8) injected intraperitoneally with a high dose of EMCV (1 × 106 PFU per mouse) and monitored for 6 d. ns, not significant (P > 0.05). (Y) The dynamic changes of acetylation and succinylation of MAVS at lysine 7 in response to VSV infection in Sirt3+/+Sirt5+/+, Sirt5−/−, Sirt3−/−, and Sirt3−/−Sirt5−/− MEF cells. MEF cells were infected with VSV for the indicated time points, and then cell lysates were immunoprecipitated with anti-MAVS antibody, followed by immunoblotting with the indicated antibodies. (Z) Quantitation of acetylated MAVS (Ac-K7-Mavs) and succinylated MAVS (Su-K7-Mavs) proteins shown in (Y). ns, not significant (P > 0.05). Data are from at least three independent experiments (mean ± SD) (B–D and G–W). |