- Title
-
Spermidine treatment: induction of autophagy but also apoptosis?
- Authors
- Watchon, M., Wright, A.L., Ahel, H.I., Robinson, K.J., Plenderleith, S.K., Kuriakose, A., Yuan, K.C., Laird, A.S.
- Source
- Full text @ Mol. Brain
|
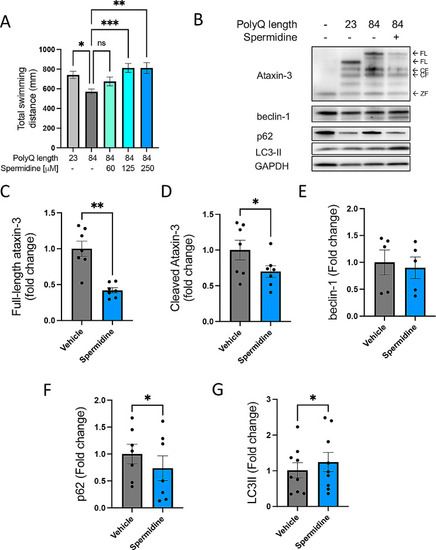
Spermidine treatment alleviates motor dysfunction in transgenic MJD zebrafish together with signs of autophagy induction. ( |
|
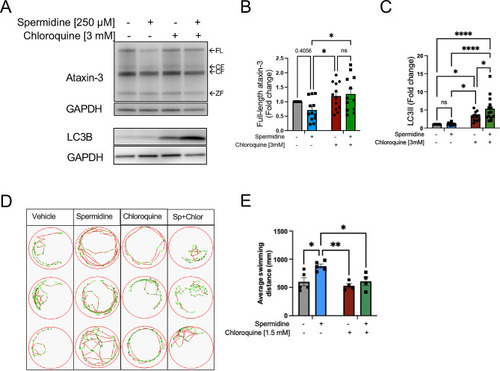
Spermidine treatment of MJD zebrafish resulted in autophagy induction and inhibition of the autophagy pathway prevented the improved swimming produced by spermidine treatment. ( PHENOTYPE:
|
|
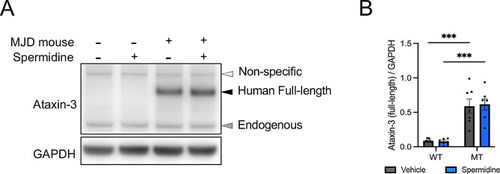
Spermidine treatment did not improve the motor impairment of CMVMJD135 mice developed motor impairment. ( |
|
Immunoblotting of wild-type and transgenic CMVMJD135 mice confirms increased expression of full-length ataxin-3 in the cerebellum of 18-week-old MJD mice. ( |
|
Spermidine treatment did not affect presence of protein aggregates within the pons and medulla of 18-week-old CMVMJD135 mice. ( |
|
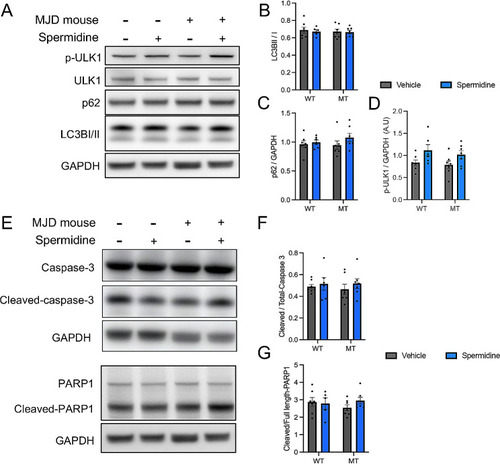
Spermidine treatment within drinking water promotes autophagy through increased phosphorylation of ULK1 in mice independent of genotype but does not induce apoptosis. ( |
|
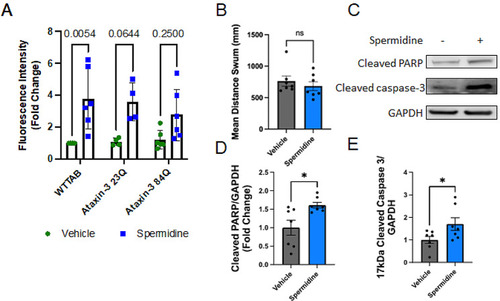
Signs of increased apoptosis in spermidine treated zebrafish larvae. ( PHENOTYPE:
|