- Title
-
The MEK-ERK signaling pathway promotes maintenance of cardiac chamber identity
- Authors
- Yao, Y., Gupta, D., Yelon, D.
- Source
- Full text @ Development
|
MEK signaling promotes ventricular identity maintenance. (A-L) In situ hybridization shows expression of amhc (A-C,J-L) or vmhc (G-I), and immunofluorescence (D-F) shows distribution of Amhc (green) within the myocardium (labeled with MF20, red), in frontal views of the heart at 48 hpf. Wild-type embryos were treated with DMSO, PD0325901 or SU5402 from 18 to 26 hpf (A-I) or from 26 to 34 hpf (J-L). In PD0325901-treated (B,E,K) and SU5402-treated (C,F,L) embryos, ectopic amhc transcripts or Amhc protein appeared in similar locations (arrowheads) within the ventricle. Additionally, PD0325901-treated (H) and SU5402-treated (I) embryos displayed reduced vmhc expression, compared with DMSO-treated controls (G). (A,B,D,F,K,L) n>13, (C) n=7, (E) n=6, (G,J) n=10, (H) n=12, (I) n=13. Scale bars: 50 µm. |
|
Ventricular regions are differentially sensitive to the inhibition of FGF signaling or MEK signaling. (A-V) Expression of amhc (A-J) or vmhc (M-V) at 48 hpf (as in Fig. 1) shows effects of treatment of wild-type embryos with a range of concentrations of SU5402 or PD0325901 from 18 to 26 hpf. Representative examples demonstrate that higher concentrations of either compound led to broader ectopic expression of amhc (A-J, arrowheads), together with reduced expression of vmhc (M-V). (K) Diagram depicts locations of ventricular regions, with darker shading in regions that more readily expressed ectopic amhc when treated with SU5402 or PD0325901. Criteria for assessing regional location of amhc expression are described in Materials and Methods. Fig. S3 provides examples of hearts with ectopic amhc in each of these regions. (L) Bar graphs show the percentage of embryos receiving each treatment that expressed ectopic amhc in each ventricular region. (A) n=21, (B) n=28, (C) n=24, (D) n=25, (E) n=29, (F,J) n=14, (G) n=7, (H) n=17, (I) n=16, (M) n=8, (N,Q,R) n=12, (O) n=6, (P,T,U) n=13, (S,V) n=15. Scale bars: 50 µm. |
|
MEK-ERK signaling activity is detectable in the myocardium. (A-D′) Three-dimensional reconstructions show pERK (red) localization within the myocardium, labeled with Tg(myl7:EGFP) (green) at 20 hpf (A,B) or CH1 (green; anti-tropomyosin) at 24 hpf (C,D). Dorsal views, anterior up (A,B) or arterial pole up (C,D). pERK was also detectable in other tissues, including the endocardium (arrowhead in A). (A'-D′) Myocardial masks (see Materials and Methods) include only the pERK labeling that overlaps with myocardial labeling. (A,B) n=17, (C,D) n=7. Scale bars: 50 µm. (E) Graph illustrates the numbers of pERK+ cardiomyocytes at 20 and 24 hpf. We often found fewer pERK+ cardiomyocytes at 24 hpf than at 20 hpf; this reduction likely reflects a more limited distribution of ERK signaling activity at 24 hpf, but we note that it could also result from differential sensitivity of the distinct laser power and exposure settings used when imaging embryos at these two stages. Data at 24 hpf include seven samples labeled with CH1 and seven samples labeled with Tg(myl7:EGFP). Red lines represent median and interquartile range. |
|
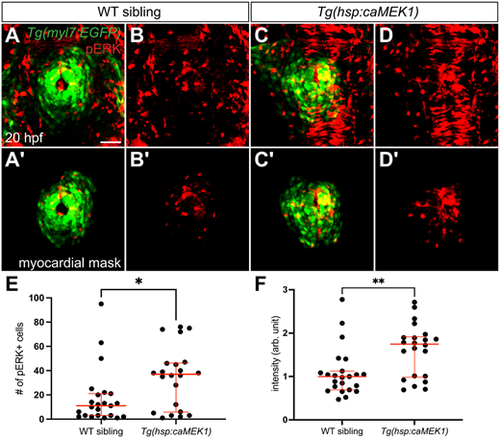
Constitutive activity of MEK1 increases pERK levels in the myocardium. (A-D′) Three-dimensional reconstructions compare pERK localization at 20 hpf (as in Fig. 3A,B) in Tg(myl7:EGFP) embryos (A,B) and Tg(myl7:EGFP) embryos carrying Tg(hsp:caMEK1) (C,D) after heat shock at 16 hpf. Myocardial masks (A′-D′) are used as in Fig. 3A′,B′. Expression of Tg(hsp:caMEK1) increased the amount of detectable pERK (C,D), compared with the endogenous level observed in wild-type (WT) sibling embryos (A,B). Although the specific distribution of pERK+ cardiomyocytes varied between Tg(hsp:caMEK1) embryos, no consistent regional pattern of pERK enrichment was apparent. (A,B) n=24, (C,D) n=22. Scale bar: 50 µm. (E) Graph (as in Fig. 3E) compares the numbers of pERK+ cardiomyocytes in WT siblings and Tg(hsp:caMEK1) embryos. Note that the number of pERK+ cells in WT siblings here was lower than that in Fig. 3E. Because of the high intensity of pERK signal in Tg(hsp:caMEK1) embryos, we reduced our laser power and exposure settings in order to avoid saturating the confocal detector; the same imaging settings were used for WT siblings and Tg(hsp:caMEK1) embryos. (F) Graph compares the average intensity of myocardial pERK signal in each embryo examined. Each data point represents the mean of the intensity mean values for the pERK signal from all of the cardiomyocytes in a WT sibling or Tg(hsp:caMEK1) embryo; normalized data are expressed in arbitrary (arb.) units (see Materials and Methods). Red lines in graphs represent median and interquartile range. *P=0.0203, **P=0.0074 (two-tailed Mann–Whitney test). |
|
MEK-ERK signaling functions downstream of FGF signaling to maintain ventricular identity. (A-D) Expression of amhc at 48 hpf (as in Fig. 1) indicates effects of heat shock at 16 hpf followed by treatment with either DMSO (A,B) or 4-5 µM SU5402 (C,D) from 18 to 22 hpf. In SU5402-treated embryos, the ventricular distribution of ectopic amhc was less broad in embryos expressing Tg(hsp:caMEK1) (D) than in WT siblings (C). (E) Graph shows percentage of SU5402-treated embryos that expressed ectopic amhc in each ventricular region. Bars represent the mean values of the percentages of embryos expressing ectopic amhc from each of the six independent experiments that were performed. Lines connect the observations in Tg(hsp:caMEK1) embryos with the WT sibling controls in each replicate, and numerals next to data points indicate the number of replicates with the same value. Asterisks near the data points of a replicate pair indicate statistically significant differences between the expression of amhc in Tg(hsp:caMEK1) and WT sibling embryos. *P<0.05, **P<0.01 (Fisher's exact test). (A) n=18, (B) n=22, (C,D) n[WT sibling, Tg(hsp:caMEK1)]=(17,18), (13,7), (15,23), (19,16), (10,13), (19,8) in six independent experiments. Scale bar: 50 µm. |
|
MEK-ERK signaling functions upstream of nkx2.5 to maintain ventricular identity. (A-D) In situ hybridization shows expression of nkx2.5 (A,B) or nkx2.7 (C,D) in the ventricular portion of the heart tube at 26 hpf, dorsal views. Wild-type embryos were treated with DMSO or PD0325901 from 18 to 26 hpf. PD0325901-treated embryos (B,D) displayed reduced expression of nkx2.5 and nkx2.7, compared with DMSO-treated controls (A,C). We note that PD0325901 treatment after 26 hpf did not appear to disrupt nkx2.5 expression (Fig. S8). (E-H) Expression of amhc at 48 hpf (as in Fig. 1) indicates effects of heat shock at 17 hpf, followed by treatment with either DMSO (E,F) or 40-50 µM PD0325901 (G,H) from 18 to 22 hpf, as well as a subsequent heat shock at 22 hpf, in WT sibling (E,G) and Tg(hsp:nkx2.5) (F,H) embryos. (I) Graph indicates percentage of PD0325901-treated embryos that expressed ectopic amhc in each ventricular region. Bars represent the mean values of the percentages of embryos expressing ectopic amhc from each of the six independent experiments that were performed. Lines connect the observations in Tg(hsp:nkx2.5) embryos with the WT sibling controls in each replicate, and numerals next to data points indicate the number of replicates with the same value. Asterisks near the data points of a replicate pair indicate statistically significant differences between the expression of amhc in Tg(hsp:nkx2.5) and WT sibling embryos. *P<0.05 (Fisher's exact test). (A,B) n=14, (C) n=15, (D,E) n=13, (F) n=19, (G,H) n[WT sibling, Tg(hsp:nkx2.5)]=(8,7), (17,13), (7,7), (15,22), (17,8), (21,10) in six independent experiments. Scale bars: 50 µm. |
|
Constitutive MEK1 activity can induce ectopic vmhc expression. (A-F) In situ hybridization shows expression of amhc (A,B) or vmhc (C-F) in frontal views at 48 hpf (A-D) or in dorsal views at 30 hpf (E,F), following heat shock at 16 hpf. In contrast to their WT siblings (C,E), most Tg(hsp:caMEK1) embryos exhibited ectopic vmhc expression in the atrium (D,F; arrowheads). (A) n=6, (B) n=10, (C) n=22, (D) n=15/21, (E) n=38, (F) n=22/32. Scale bars: 50 µm |
|
Constitutive MEK1 activity induces ectopic vmhc expression in the atrial myocardium. (A-R) Fluorescent in situ hybridization shows expression of vmhc (green) together with immunofluorescence using MF20 (red, A-I) or labeling Amhc (red, J-R) at 48 hpf, following heat shock at 16 hpf. Three-dimensional reconstructions of frontal views (A-F,J-O), as well as single optical sections (G-I,P-R), highlight the localization of vmhc expression within the atrial myocardium in embryos expressing Tg(hsp:caMEK1) (D,E,G,I,M,N,P-R; arrowheads). In these experiments, all examined Tg(hsp:caMEK1) embryos exhibited ectopic vmhc, in contrast to the incomplete penetrance documented in Fig. 7; this difference is likely due to the heightened sensitivity of fluorescent in situ hybridization. (A-C) n=6, (D-I) n=8/8, (J-L) n=5; (M-R) n=10/10. Scale bars: 50 µm. |