- Title
-
The zebrafish paralog six2b is required for early proximal pronephros morphogenesis
- Authors
- Belcher, B., Vestal, J., Lane, S., Kell, M., Smith, L., Camarata, T.
- Source
- Full text @ Sci. Rep.
|
Early expression of EXPRESSION / LABELING:
|
|
Pronephric marker expression following injection of |
|
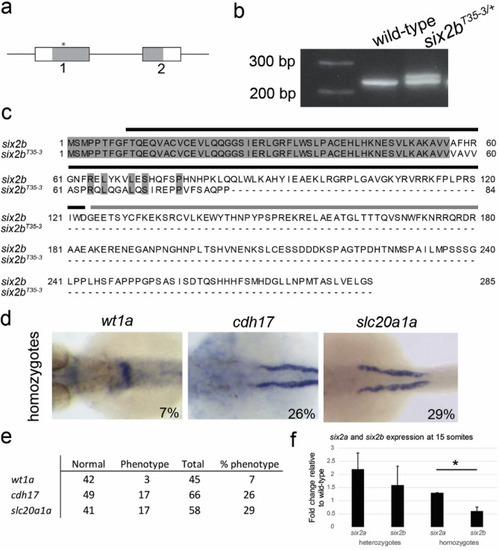
Stable CRISPR/Cas9 mutagenesis of EXPRESSION / LABELING:
PHENOTYPE:
|
|
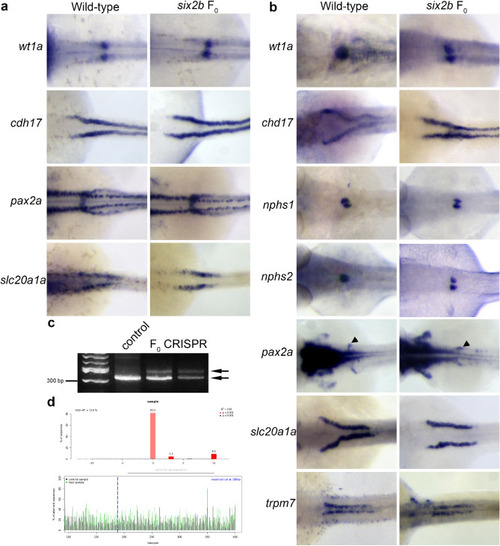
Pronephric marker expression in F0 embryos following |