- Title
-
GCN5L1 regulates pulmonary surfactant production by modulating lamellar body biogenesis and trafficking in mouse alveolar epithelial cells
- Authors
- Xu, W., Ma, X., Wang, Q., Ye, J., Wang, N., Ye, Z., Chen, T.
- Source
- Full text @ Cell Mol. Biol. Lett.
|
GCN5L1 gene KO impaired surfactant production in MLE-12 cells. |
|
Disruption of GCN5L1 altered the expression of Cebpα and surfactant-related genes. |
|
Disruption of GCN5L1 altered the activity of the ROS–ERK–FOXO1 axis in MLE-12 cells. |
|
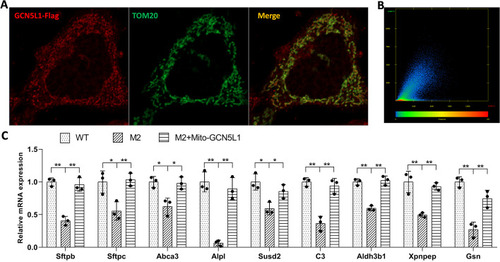
Mitochondria-localized GCN5L1 is responsible for the regulation of surfactant-related genes. |
|
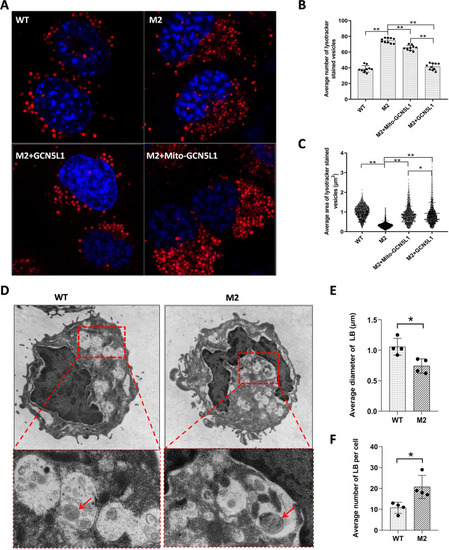
Impairment of LB positioning/trafficking in GCN5L1-KO cells. |
|
Abnormal size and number of LB-like organelles in GCN5L1-KO cells. |
|
Localization of GCN5L1 in LBs. Cells were transfected with GCN5L1–FLAG-expressing plasmid and stained with anti-Lamp1 and anti-FLAG antibodies |
|
Accumulation of SP proteins in GCN5L1-KO cells. |
|
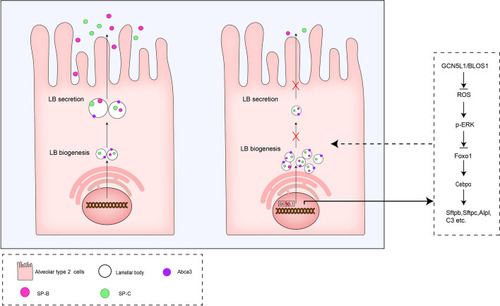
A hypothetical illustration of GCN5L1 functions in the regulation of pulmonary surfactant production |