- Title
-
The ndrg2 Gene Regulates Hair Cell Morphogenesis and Auditory Function during Zebrafish Development
- Authors
- Wang, C., Wang, X., Zheng, H., Yao, J., Xiang, Y., Liu, D.
- Source
- Full text @ Int. J. Mol. Sci.
|
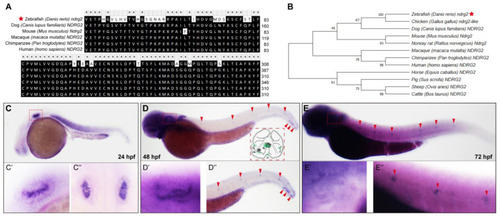
The |
|
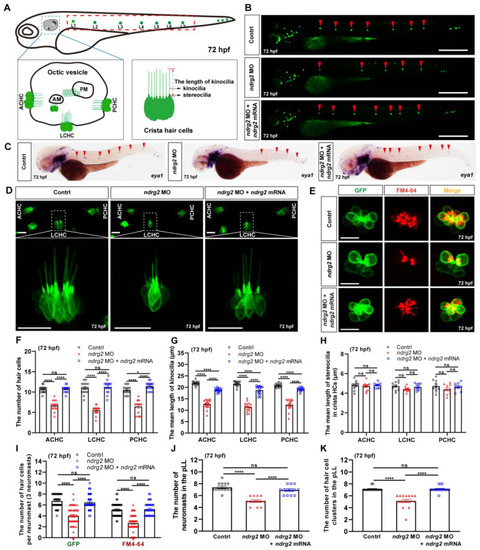
Knockdown of the |
|
Knockdown of the |
|
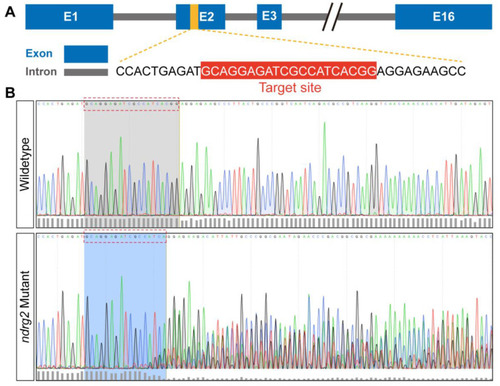
CRISPR/Cas9-mediated |
|
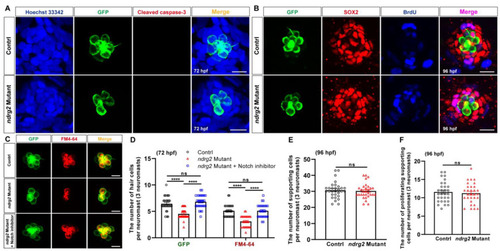
Knockout of the |
|
Loss of the |