- Title
-
Long-term in toto Imaging of Cellular Behavior During Nerve Injury and Regeneration
- Authors
- Tian, W., González-Suarez, A., Lopez-Schier, H.
- Source
- Full text @ Bio Protoc
|
Main tools for the protocol. A. Photograph exemplifying the placement of a plastic Petri dish containing eggs into an incubator. B. Photograph of a standard Petri dish, glass pipette with a hair loop attached to its tip, a forceps and a plastic transfer pipette. C. Example of a stereomicroscope with a typical microinjection apparatus. D. Photograph of an inverted spinning-disc microscope for long-term imaging. |
|
Procedures for collecting eggs and raising specimens. A. Photograph of a breeding tank, with a bottom grid to enable eggs to fall outside the reach of adult fish (which may otherwise eat them), a Petri dish and an egg-harvesting strainer. B. Once the inner container is removed with the breeding adults, the water with the eggs contained in the breeding tank is gently poured through the strainer to collect eggs. C. The eggs are gently and briefly washed with clean water from the facility system. D. The strainer is then placed inside down onto the inner (bottom) part of the Petri dish and eggs are flushed into this dish applying a gently flow of water using a plastic bottle. E. The eggs will now be visible in the dish. F. The dish with the eggs can then be moved to a stereomicroscope of fluorescence |
|
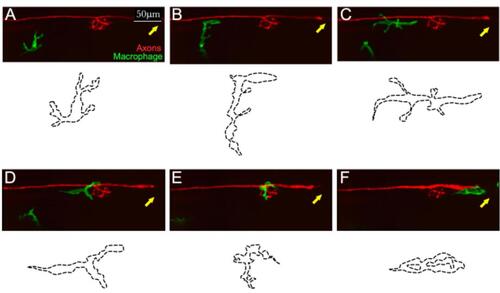
Example of in toto videomicroscopy of axons and macrophages. A–F. Still images from a high-resolution time-lapse recording of a macrophage (green) and a lateralis sensory nerve (red) in the trunk of a larval zebrafish. A single macrophage is seen migrating towards the nerve immediately after it has been severed with a laser. A second macrophage is seen below, which never makes contact with the nerve. Over time, the macrophage moves along the nerve, surveilling damage (which can be seen as a discontinuity of the nerve on the left-hand side of the panels (C and D). The drawings below each panel (dotted lines) describe the two-dimensional shape changes ofthis single macrophage during the course of videomicroscopic imaging. Scale bars is 50 μm. |
|
Example of in toto videomicroscopy of axons and Schwann cells. A–C. Fluorescence images from a high-resolution time-lapse recording of the movement of Schwann cells (green) and the regeneration of a lateralis afferent nerve (red) after transection. A shows the merge of the two fluorescence channels, labelling the sensory nerve (Red) and the Schwann cells (green).The respective individual channels are show in B and C. D shows the tracks of the moving Schwann cells. t1, t2, t3, t4, t5, t6 indicate different time points extracted from the time-lapse. Dt6. It a zoom-in of the entire track of Schwann cells of at t6. |
|
Example laser-mediated nerve transection. A-1 and A-2. Screenshots from the transection, before and after laser-mediated wounding, respectively. Images show the VISION software settings used during this procedure, as well as the targeted region within the nerve axon (yellow rectangle). A-1.1 and A2.1. Spinning-disk images render the nerve axon targeted by the bean laser (yellow rectangle), from before and after transection. |