FIGURE SUMMARY
- Title
-
ErCas12a and T5exo-ErCas12a Mediate Simple and Efficient Genome Editing in Zebrafish
- Authors
- Han, B., Zhang, Y., Zhou, Y., Zhang, B., Krueger, C.J., Bi, X., Zhu, Z., Tong, X., Zhang, B.
- Source
- Full text @ Biology (Basel)
|
Figure 1. ErCas12a mediates efficient MMEJ-based knockin at the zebrafish tyr locus. (A) Schematic diagram of reporter gene knockin mediated by the MMEJ pathway through ErCas12a or Cas9 targeting at the tyr locus. The sequences of the overlapping target sites are displayed, with PAM sequences indicated in blue characters. (B) Indel efficiency of tyr ErCas12a site in embryos injected with ErCas12a mRNA and tyr pre-crRNA following 1 h heatshock at different developmental stages. (C) Comparison of indel efficiency of tyr Cas9 site with ErCas12a site with or without 1 h 34 °C heatshock at 3–4 hpf. HS: heatshock. Data in (B,C) represent mean ± s.d. of at least three independent replicates. Unpaired two-tailed Student’s t-test was used to calculate p values (* p < 0.05; ** p < 0.01; *** p < 0.001; “n.s.” indicates the difference is not significant). (D) Representative fluorescence expression of tyr knockin F0 embryos obtained by injection of either ErCas12a or Cas9 MMEJ systems. Embryos with red fluorescence were separated into broad (Class I) and sparse (Class II) groups according to the expression pattern. Embryos with no fluorescence were designated Class III. The embryos are in lateral view with anterior to the left. Scale bar: 200 μm. (E) Evaluation of MMEJ-based knockin efficiency by the proportion of broad (Class I) and sparse (Class II) fluorescence-positive embryos injected with ErCas12a or Cas9 MMEJ-based knockin system at the tyr locus. ErCas12a system injected embryos were separated into heatshocked (ErCas12a HS) and control (ErCas12a) groups. Number of embryos evaluated (n) is shown for each condition. Chi-square test was used to calculate p values between heatshocked (ErCas12a HS) group and others (** p < 0.01; **** p < 0.0001).
|
|
Figure 2. Evaluation of germline transmission of ErCas12a-mediated knockin with the MMEJ donor at the tyr locus. (A) Representative images of the red fluorescent reporter expression of F1 embryos obtained from outcross of the three tyr MMEJ knockin-positive F0 adults listed in Table 1. Red fluorescence can be observed in the eyes and pigment cells of the embryos. Scale bar: 200 μm. (B) Junction PCR results of the tyr knockin F1 embryos showing red fluorescence from F0 #3. Primers tyr F1 and tdt R were used to amplify the 5′ junction, and primers bF1 and tyr R1 were used to amplify the 3′ junction. (C) Sequencing alignment of the junction PCR products from (B) after TA cloning. Black characters indicate sequence of the tyr WT allele, blue characters represent the homologous sequence designed in the donor, and red characters represent the donor sequence.
|
|
Figure 3. ErCas12a mediates efficient NHEJ-based knockin at the zebrafish tbx2b locus. (A) Schematic diagram of R295H mutation and reporter gene knockin mediated by the NHEJ pathway through ErCas12a or Cas9 targeting at the tbx2b locus. Target site sequences are displayed, with PAM sequences indicated in blue. (B) Comparison of indel efficiency of tbx2b Cas9 site with ErCas12a site with or without 1 h heatshock at 34 °C. HS: heatshock. Data represent mean ± s.d. of at least three independent replicates. Unpaired two-tailed Student’s t-test was used to calculate p values (*** p < 0.001; “n.s.” indicates the difference is not significant). (C) Representative fluorescence expression of tbx2b knockin F0 embryos obtained by injection of either ErCas12a or Cas9 NHEJ systems. Embryos with red fluorescence were separated into broad (Class I) and sparse (Class II) groups according to the expression pattern. Embryos with no fluorescence were designated Class III. The embryos are in lateral view with anterior to the left. Arrows indicate the regions showing red fluorescent signal. Scale bar: 200 μm. (D) Evaluation of NHEJ-based knockin efficiency by the proportion of broad (Class I) and sparse (Class II) fluorescence-positive embryos injected with ErCas12a or Cas9 systems at the tbx2b locus. The ErCas12a system injected embryos were separated into heatshocked (ErCas12a HS) and control (ErCas12a) groups. Number of embryos evaluated (n) is shown for each condition. Chi-square test was used to calculate p values between heatshocked (ErCas12a HS) group and others (**** p < 0.0001).
|
|
Figure 4. Evaluation of germline transmission of ErCas12a-mediated knockin with the NHEJ donor at the tbx2b locus. (A) Fluorescence reporter expression of F1 embryos obtained from outcross of a tbx2b NHEJ knockin F0 adult. Red fluorescence can be observed in the nervous system, eyes, pectoral fins, and endocardium of the F1 embryos. Scale bar: 200 μm. (B) Junction PCR results of the tbx2b knockin F1 embryos bearing red fluorescence. Primers tbx2b F1 and tbx2b R1 were used to amplify the 5′ junction, and primers bF2 and tbx2b R1 were used to amplify the 3′ junction. Note that due to the structure of the knockin allele, the 5′ junction amplicon of the of knockin allele is indistinguishable from that of the WT allele by electrophoresis. (C) Sequencing alignment of the junction PCR products after TA cloning. As the 5′ junction PCR product was a mixture of amplicons from the WT and knockin alleles, only sequencing results with indel mutations in the intronic junction site were considered to be amplified from the knockin allele. Blue characters indicate the tbx2b WT allele, red characters represent the donor sequences, and black characters represent random insertions resulting from NHEJ repair. Underlined characters indicate the PAMs of the tbx2b ErCas12a site and lamgolden Cas9 site for donor linearization.
|
|
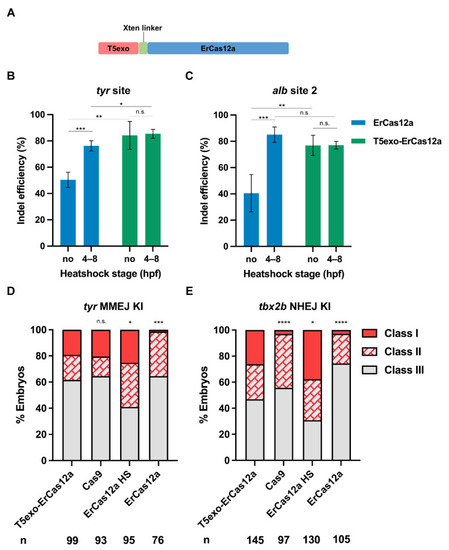
Figure 5. T5exo-ErCas12a efficiently mediates heatshock-free indel mutation and large fragment knockin. (A) Schematic diagram of ErCas12a fused with T5 exonuclease at the N-terminus. The two proteins are separated by an Xten linker. (B) Indel efficiency in embryos injected with T5exo-ErCas12a mRNA and tyr pre-crRNA under heatshock or non-heatshock conditions. The results were compared with ErCas12a mRNA injection under the same conditions. (C) Indel efficiency in embryos injected with T5exo-ErCas12a mRNA and alb pre-crRNA2 under heatshock or non-heatshock conditions. The results were compared with ErCas12a mRNA injection under the same conditions. Data in (B,C) represent mean ± s.d. of at least three independent replicates. Unpaired two-tailed Student’s t-test was used to calculate p values (* p < 0.05; ** p < 0.01; *** p < 0.001; “n.s.” indicates the difference is not significant). (D) Evaluation of MMEJ-based knockin efficiency by the proportion of broad (Class I) and sparse (Class II) fluorescence-positive embryos injected with T5exo-ErCas12a, Cas9, or ErCas12a (with and without heatshock) systems at the tyr locus. Representative images of different expression patterns are shown in Figure 1D. HS: Heatshock. (E) Evaluation of NHEJ-based knockin efficiency by the proportion of broad (Class I) and sparse (Class II) fluorescence-positive embryos injected with T5exo-ErCas12a, Cas9, or ErCas12a (with and without heatshock) systems at the tbx2b locus. Representative images of different expression patterns are shown in Figure 3C. HS: Heatshock. Number of embryos evaluated (n) is shown for each condition. Chi-square test was used to calculate p values between T5exo-ErCas12a group and others in (D,E) (* p < 0.05; **** p < 0.0001; “n.s.” indicates the difference is not significant).
|
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Biology (Basel)