- Title
-
Medium-throughput zebrafish optogenetic platform identifies deficits in subsequent neural activity following brief early exposure to cannabidiol and Δ9-tetrahydrocannabinol
- Authors
- Kanyo, R., Amin, M.R., Locskai, L.F., Bouvier, D.D., Olthuis, A.M., Allison, W.T., Ali, D.W.
- Source
- Full text @ Sci. Rep.
|
High-throughput quantification of neural activity in freely-swimming zebrafish larvae. ( |
|
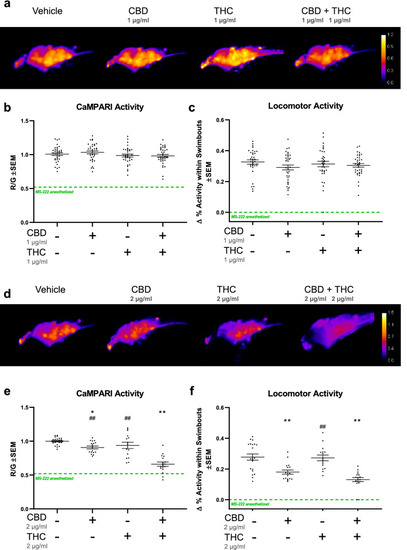
Early application of CBD and THC reduce subsequent neural activity and locomotor activity. ( |
|
Antagonistic effect of CBD on neural activity is enhanced when combined with sub-effective doses of THC. Zebrafish larvae were exposed to a series of CBD and THC concentrations by themselves or in combination, which are mostly sub-effective, or in the case of CBD, were minimally effective. ( |
|
Cannabinoid receptor 1 (CB1R) and CB2R are both involved in CBD-mediated reduction of locomotor activity. Zebrafish larvae were exposed to CBD (3 µg/ml) with CB1R- and CB2R inhibitors, AM251 and AM630, respectively. ( |