- Title
-
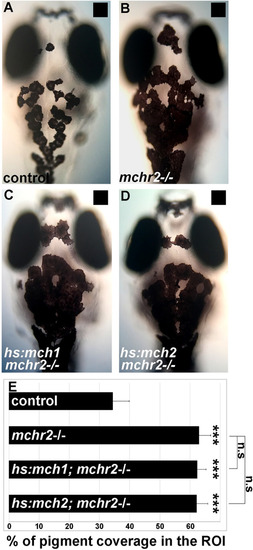
Genetic deciphering of the antagonistic activities of the melanin-concentrating hormone and melanocortin pathways in skin pigmentation
- Authors
- Madelaine, R., Ngo, K.J., Skariah, G., Mourrain, P.
- Source
- Full text @ PLoS Genet.
|
|
|
|
|
PHENOTYPE:
|
|
|
|
|
|
|
|
PHENOTYPE:
|
|
|
|
|
|
|
|
|
|
|