- Title
-
The Reissner Fiber in the Cerebrospinal Fluid Controls Morphogenesis of the Body Axis
- Authors
- Cantaut-Belarif, Y., Sternberg, J.R., Thouvenin, O., Wyart, C., Bardet, P.L.
- Source
- Full text @ Curr. Biol.
|
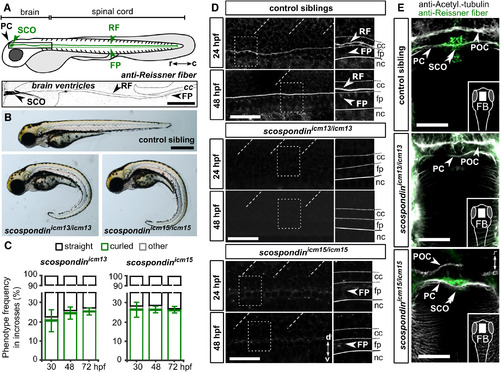
Mutations in scospondin Lead to the Absence of the Reissner Fiber and Defects in Body Axis Formation (A) The Reissner fiber (RF) is localized in posterior ventricles of the brain and spinal central canal. At embryonic stages, SCO-spondin is secreted in the cerebrospinal fluid from the sub-commissural organ (SCO) below the posterior commissure (PC) and from the floor plate (FP) to form the fiber. Top: a scheme based on several immunohistochemistry experiments (below). Bottom: Z projection of a stack of a few lateral optical sections of the brain ventricles and rostral central canal (cc) of a 72 hpf embryo immunostained against the Reissner fiber (arrow). c, caudal; r, rostral. Scale bar represents 100 μm. (B) 72 hpf scospondinicm13/icm13 and scospondinicm15/icm15 larvae showing curled-down phenotypes. Scale bar represents 500 μm. (C) Proportion of curled-down phenotype over developmental time in both scospondin allele incrosses (mean ± SEM; n = 386 and 248 embryos for scospondinicm13/icm13 and scospondinicm15/icm15, respectively, from three independent clutches). The abnormal curvature of the body axis is detected from 30 hpf onward. Gastrulation-defective embryos were negligible (“other,” gray). (D) Z projection of a stack of lateral optical sections (depth 4–5 μm) of the spinal cord immunostained against the Reissner fiber in control and scospondin mutants at 24 hpf (top) and 48 hpf (bottom). Both mutants are deprived of the Reissner fiber in the central canal from 24 hpf onward but immunoreactivity is detected in the floor plate of scospondinicm15/icm15 (see insets on right panels highlighting the dotted-box regions) (n = 33; 63 control embryos, n = 7; 19 scospondinicm15/icm15 embryos, n = 12; 33 scospondinicm13/icm13 at 24; 48 hpf, respectively). d, dorsal; nc, notochord; v, ventral. Scale bars represent 40 μm. (E) Z projection of stacks of dorsal optical sections (depth 23–26 μm) of 48 hpf forebrains (FBs) immunostained for acetylated tubulin (gray) and the Reissner fiber (green) show Reissner fiber material in SCO (double arrowheads) of control and scospondinicm15/icm15 embryos but not scospondinicm13/icm13. Arrows indicate axonal commissures. POC, post-optic commissure. Scale bars represent 30 μm. See also Figure S1 and Table S1. PHENOTYPE:
|
|
Structural and Dynamic Properties of Cilia Appear Intact in the scospondinicm13/icm13 Mutant (A) Z projection of stacks of lateral optical sections (depth 4–5 μm) of spinal cord immunostained against acetylated tubulin show intact cilia projecting into the central canal of control and scospondinicm13/icm13 larvae at 24 (top) and 30 hpf (bottom). In comparison, elipsa embryos exhibit fewer cilia at 24 hpf, which are not maintained at 30 hpf. Scale bars represent 15 μm. (B) Time projection from a 30-s-long time series acquired at 17 Hz and indicating movement of cilia expressing GFP in Tg(β-actin:Arl13b-GFP; scospondinicm13/icm13) animals (right) and control siblings (left). Note the similarity in position (posterior tilt, dashed lines) and beating amplitude of motile cilia in the central canal (arrowheads) in mutant embryos compared to control siblings. The schematic summarizes our observations. Rostral, left; dorsal, top. Scale bar represents 10 μm. See also Figure S2 and Video S1. |
|
Cerebrospinal Fluid Properly Flows in the Central Canal of the scospondinicm13/icm13 Mutant (A and B) Lateral view of the central canal (A; transmitted, top) filled with fluorescent beads (bottom). Scale bar represents 30 μm. Time-lapse images at two positions (dorsal and ventral) are represented with the rostro-caudal axis as the horizontal axis and time as the vertical one (B). Kymographs reveal a bidirectional flow, with bead trajectories pointing at opposite directions in the dorsal and ventral central canal. Bead trajectories (green) were used to estimate bead velocities along the rostro-caudal axis. (C) Bead velocities were similar in control siblings (black) and scospondinicm13/icm13 (green) embryos in the dorsal and ventral central canal (control: n = 570; 584; mutants: n = 582; 634 trajectories in the dorsal; ventral position, respectively; p = 0.07 and 0.33, t = −1.8 and 0.99, and degrees of freedom [df] = 28.8 and 16 in the dorsal and ventral position, respectively, two-tailed t test). Values are given as median ± interquartile range; one boxplot per fish; color intensity reflects the number of measured trajectories per fish. ns, not significant. (D) 30 hpf zebrafish embryo injected with fluorescent beads in brain ventricles shows transport down the central canal (inset) 30 min post-injection (mpi), reaching here the 8th somite (arrowhead). FBV, forebrain ventricle; HBV, hindbrain ventricle. Scale bar represents 0.5 mm. (E) The fluorescence front moving down the central canal over time was indistinguishable in control siblings (gray) and scospondinicm13/icm13 mutants (green). As a consequence, the fluorescence reached the same level 1 hr after injection (n = 6 versus 7, respectively, p = 0.83, t = −0.21, df = 10.3, two-tailed t test). Error bars are mean ± SEM. See also Figure S3 and Video S2. PHENOTYPE:
|
|
Intact Cilia Are Necessary for the Formation of the Reissner Fiber in the Central Canal (A) Z projection of stacks of lateral optical sections (depth 4–5 μm) of spinal cord at 48 hpf showing modifications of immunoreactivity for the Reissner fiber in mutants with defective cilia (iguana, oval, elipsa, and kurly) compared to control siblings, where a continuous fiber runs along the entire central canal. d, dorsal; v, ventral. Scale bar represents 30 μm. (B) Zoom of regions boxed in (A) showing depositions of the Reissner material in the central canal. Deposits occur as continuous densely packed fiber (1), continuous unpacked fiber (2), discontinuous loosely packed material (3), aggregated material (4), diffuse material (5), and absence of material (6). cc, central canal; fp, floor plate; nc, notochord. (C) Distribution of defects in the Reissner material along the rostro-caudal axis and for each mutant displayed as mean segment number ± SEM (n = 24; 11; 6; 8; 5 embryos for control siblings; iguana; oval; elipsa, and kurly, respectively). (D and E) Before elipsa and kurly embryos develop the curled-down phenotype (24 hpf embryo), defects in the Reissner material can be observed in the central canal. Right panels (D) show zoom from regions boxed in left panels with depositions of the Reissner material in the central canal; the numbers correspond to the equivalent defects at 48 hpf (depicted in B and C). Distribution of defects in the Reissner material along the rostro-caudal axis for the two mutants at 24 hpf (E) (bottom, n = 12 and 19 embryos for elipsa and kurly, respectively) compared to control siblings (top; n = 28). Scale bar represents 30 μm. Cc, central canal; fp, floor plate; nc, notochord. See also Figure S4. |
|
Embryonic posterior axis defects strictly correlate with scospondin mutated alleles, related to Figure 1 and Table S1 A. CRISPR/Cas9-mediated genome editing leading to a nonsense mutation, and a five amino acids insertion in the EMI domain of SCO-spondin for the scospondinicm13 and scospondinicm15 allele, respectively (SP: signal peptide, EMI: Emilin domain, SCOR: SCO-spondin repeats, vWD: von Willebrand D domain, LDLRa: Low-Density Lipoprotein Receptor type A Repeat, TSR: Thrombospondin type 1 Repeats, CTCK: C-terminal Cystine Knot, see [S1]). B. scospondin mutants show different levels of severity in body axis curvature at 48 hpf (score 1, 2, 3). Curvature defects are classified according to the angle (θ) between the tail and the rostral axis. C. Around 25% of embryos showed curvature defects from 30 hpf onwards, in agreement with Mendelian distributions. Curvature defects (%, mean ± SEM) were more pronounced over time for both scospondin alleles (from 30 to 72 hpf, n=386 and 248 embryos for icm13 and icm15 alleles respectively, n= 3 clutches). D. Embryos from scospondinicm13/+ and scospondinicm15/+ incrosses were genotyped at 72 hpf using the Sph1 restriction site loss in scospondin mutants. E. All scospondin homozygous mutants showed the curled-down phenotype and it was observed neither in the scospondin heterozygous nor in wild type embryos. The same is true for trans-heterozygous scospondinicm13/icm15 embryos (data not shown). F. To perform morphometric analysis of scospondin mutants, 48 hpf control and curled-down embryos from both alleles were measured for the eye, brain ventricles and tail area (dotted lines) as well as head and trunk height and tail length (solid lines). (Scale bar represents 1 mm.) G. To perform morphometric measurements at the level of the trunk, 30 hpf control and scospondinicm13/icm13 embryos injected at one cell stage with the Ras-eGFP mRNA were immunostained against GFP. The membrane-tag fluorescence was used to measure the spinal cord (sc), floor plate (fp) and notochord (nc) height in both straight and curled-down siblings. H. Representative images of a 30 hpf control embryo immunostained against phospho-histone 3 (PH3, right) allowing to detect cell proliferation. DAPI counterstaining of the nuclei (left) allowed differentiating dorsal and ventral tissues above and under the floorplate (dotted line) respectively. I. Quantification of PH3 positive cells in control (black) and scospondinicm13/icm13 (green) embryos at 30 hpf in dorsal and ventral tissues. n=10 embryos for each condition. p= 0.57; 0.55, t= -0.58; 0.61 and df= 15; 14 for dorsal; ventral tissues respectively; two-tailed t-test. Scale bars represent 30 μm in G and H. |
|
Cilia length and density are unchanged in scospondinicm13/icm13 mutants, related to Figure 2 and Video S1 A. Z projection of a stack of lateral optical sections (depth= 3 μm) of the spinal cord of a 30 hpf embryo immunostained against Acetylated-tubulin (left) and after tracing to estimate cilia length (yellow). Scale bar represents 10 μm. B. Cilia length is similar in scospondinicm13/icm13 embryos (green) compared to control siblings (black). (Median ± interquartile range, n= 83; 105; 95 and 95 and 67; 96; 94 and 89 cilia at 24; 30; 35 and 40 hpf for control and scospondinicm13/icm13; p = 0.53; 0.14; 0.53 and 0.61, respectively. t= -0.7; -1.8; -0.67 and -0.55, respectively; df = 3.05; 3.4; 3.9 and 2.9, respectively, two-tailed t-test). Each box plot represents a single fish; color intensity reflects the number of measured objects for each fish. Similar results were obtained for scospondinicm15/icm15 embryos (data not shown). C, D. Z projection of stacks of lateral optical sections (depth = 3 μm) of the spinal cord stained with DAPI and against Gamma-tubulin in a 30 hpf control sibling (top) and scospondinicm13/icm13 curled-down embryo (bottom), allowing to detect basal bodies around the central canal (cc). Centers of mass of detected objects were used to quantify cilia density shown in D as the number of basal bodies per 100 μm2. n= 5 control (black) and 5 scospondinicm13/icm13 embryos (green). p= 0.52, t= 0.68, df= 8, two-tailed t-test. Each point corresponds to a single fish. Scale bars represent 10 μm in C. |
|
Transport of exogenous fluorescent beads in the cerebrospinal fluid is abolished in elipsa mutants with defective cilia, related to Figure 3 and Video S2. A. Superimposed images of transmitted light (DIC) and fluorescent 20-nm diameter beads (magenta) injected in the third ventricle of 30 hpf control sibling and elipsa mutant embryos. Beads are transported down the central canal in straight control siblings, but not in elipsa mutants as shown 60 minutes post-injection (mpi). Scale bar represents 0.5 mm. The progression of the fluorescence front in the central canal is quantified in B as the segment number reached 60 minutes after injection (mean ± SEM) in control sibling (n = 3) and elipsa mutant embryos (n = 5) (p = 0.0014; t = 12.46, df = 2.84, two tailed t-test). |
|
The sub-commissural organ is immunoreactive for the Reissner fiber material in mutants with defective cilia, related to Figure 4. Z projection of stacks of dorsal optical sections (depth = 23 - 26 μm) of 48 hpf embryos immunostained against Acetylated-tubulin (magenta) allowing to detect axonal tracts (POC: post-optic commissure, PC: posterior commissure, arrowheads), and the Reissner fiber material (green) in the sub-commissural organ (SCO, double arrowheads). Control embryos (here an iguana sibling) as well as iguana, oval, elipsa and kurly mutants show immunoreactivity for the Reissner fiber material in the SCO. Rostral, top. e: eye. Scale bar represents 50 μm. |