- Title
-
The zebrafish as a model system for analyzing mammalian and native α-crystallin promoter function.
- Authors
- Posner, M., Murray, K.L., McDonald, M.S., Eighinger, H., Andrew, B., Drossman, A., Haley, Z., Nussbaum, J., David, L.L., Lampi, K.J.
- Source
- Full text @ Peer J.
|
Confocal imagery showing representative sites of GFP expression produced by mouse and zebrafish α-crystallin promoters. Examples of lens expression produced with a zebrafish αA promoter (A and B). Various sites of extraocular expression shown as single z-planes (on left) and as 3-dimensional renders (on right) for skeletal muscle produced with a mouse αB promoter (C and D); for notochord produced with a zebrafish αBb promoter (E and F); dorsal to the yolk produced with a zebrafish aA promoter (G and H). |
|
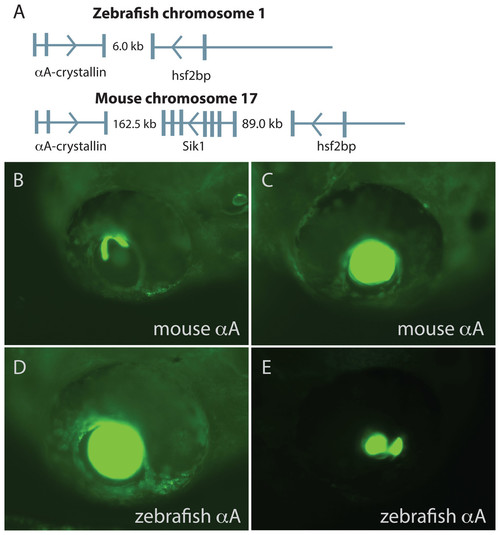
Comparison of mouse and zebrafish αA-crystallin chromosomal arrangement and their ability to drive GFP expression in zebrafish embryos. The structural and functional conservation of mammalian and zebrafish αA-crystallin is mirrored in their shared syntenic relationship with hsf2bp (A). Vertical bars note exons, thin horizontal lines note introns and arrows show direction of transcription. The promoter regions for each gene produced similar temporal and spatial expression patterns (B–E), with expression almost exclusively restricted to the lens. The extent of lens expression varied for both orthologous promoters (compare B to C for mouse and D to E for zebrafish). |
|
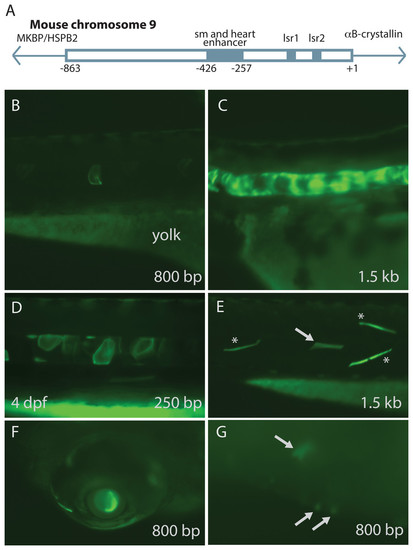
Mouse αB-crystallin promoter fragments produced native expression in zebrafish embryos. Enhancer elements of a promoter upstream of mouse αB-crystallin were previously shown to regulate expression in skeletal muscle (sm), heart and lens (lsr1 and 2) (A; adapted from Swamynathan & Piatigorsky, 2002). Fragments containing 250 bp, 0.8 and 1.5 kb lengths of this promoter produced GFP expression in zebrafish embryo notochord (B–D), skeletal muscle (E), lens (F) and heart (G; arrows). (E) shows GFP expression in both fast (noted by *) and slow twitch (noted by arrows) muscle fibers. The yolk remaining in these embryos is autofluorescent. The 250 bp fragment, which lacked the heart and skeletal muscle enhancer, produced less frequent GFP expression in these tissues, and GFP expression onset was slightly earlier (Tables 3 and 4). |
|
The paralogous zebrafish αBa- and αBb-crystallin promoters produced similar, but distinct, GFP expression profiles. Zebrafish αBb-crystallin has the same syntenic relationship with Hspb2 as mouse αB-crystallin, although the intergenic region between the two genes is much larger in the zebrafish (A). The zebrafish αBa-crystallin paralog has moved to a separate chromosome. Both zebrafish paralogs produced GFP expression most often in notochord (B) and skeletal muscle (C). The αBa paralog drove expression in these tissues equally while αBb was more active in skeletal muscle (D). Expression in lens (E) and extralenticular regions of the eye was more rare. Images shown are representative with the details of GFP expression not differing noticeably between paralogs or the promoter length used. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |