- Title
-
AmotL2 integrates polarity and junctional cues to modulate cell shape
- Authors
- Hultin, S., Subramani, A., Hildebrand, S., Zheng, Y., Majumdar, A., Holmgren, L.
- Source
- Full text @ Sci. Rep.
|
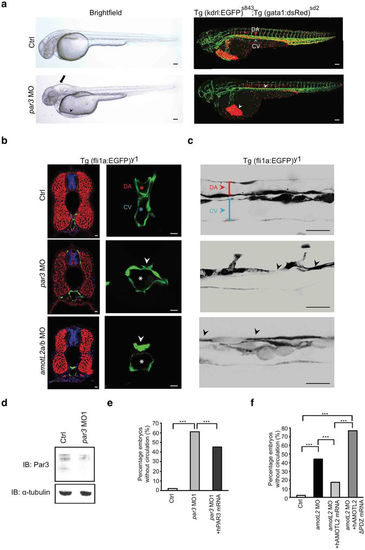
Par3 is required for aortic lumen formation during zebrafish development. (a) Brightfield (left) and fluorescence (right) images of double transgenic Tg (kdrl:EGFP)s843;Tg (gata1:dsRed)sd2, control (top) and par3 MO (bottom) injected embryos at 48hpf. The par3 morphants exhibited pericardial (arrowhead) and brain (arrow) edema. In addition, they lacked circulation, and no erythrocytes could be observed in the trunk vasculature (arrowhead). Scale bar, 100 μm. (b) Transverse sections of 48hpf Tg(fli1a:EGFP)y1 control (top row) and par3 (mid row) morphants. Sections were stained with phalloidin for F-actin (red) and TO-PRO-3 iodide to visualize nuclei (blue). In control embryos patent lumens could be observed in both the dorsal aorta (red asterisk) and cardinal vein (blue asterisk). The par3 morphants showed narrow aortic lumens with present constrictions (arrowhead), while the vein was lumenized (white asterisk). As a comparison sections of amotL2a/b embryos were also included (bottom row). Note the similarity of the aortic phenotype. Scale bar, 10 μm. (c) Sagittal view of the DA (red bracket/ arrowhead) and PCV (blue bracket/ arrowhead) in control, par3 and amotL2 morphants. Both amotL2 and par3 morphants show DA constrictions (arrowheads) and reduced DA diameter. Scale bar, 50 μm. (d) Western blot analysis showing the knock-down efficiency of the par3 MO1. Alpha-tubulin was used to control for equal loading. (e) Quantification of the circulation defect in the par3 morphants. The phenotype could be partially rescued by co-injecting the morpholino with a human PAR3 mRNA. N(ctrl) = 147 embryos, N(par3 MO) = 154 embryos, N(par3 MO + hPAR3 mRNA) = 117 embryos. *** p ≤ 0.001. (f) Rescue experiment of the circulatory phenotype observed in the amotL2a/b MO zebrafish embryos. The circulation could be restored by co-injecting the morpholinos with a human AMOTL2 mRNA, but not with an AMOTL2 mRNA lacking the c-terminal PDZ-binding motif. N(ctrl) = 100 embryos, N(AmotL2 MO) = 111 embryos, N(AmotL2 MO + hAMOTL2 mRNA) = 141 embryos, N(AmotL2 MO + hAMOTL2 ΔPDZ mRNA) = 66 embryos, *** p ≤ 0.001. PHENOTYPE:
|
|
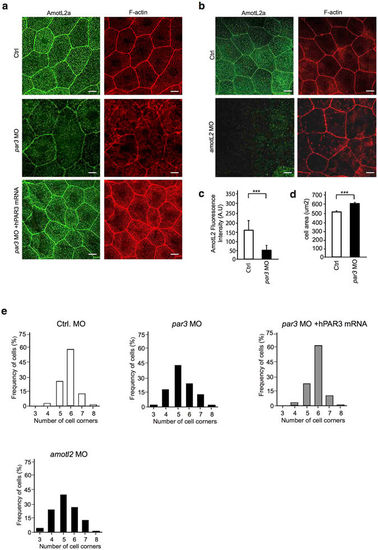
Par3 controls actin filament architecture and morphology in epithelial cells of zebrafish skin. (a) Immunofluorescence staining of AmotL2a (green) and F-actin (red) in skin of control (first panel) and par3 MO (second panel) treated zebrafish embryos at 34hpf. In control embryos, AmotL2a localizes to cell-cell junctions, as well as actin positive apical microridges. In the absence of Par3 those actin structures are lost and in addition AmotL2a expression at cell-cell junctions is reduced. The actin filament phenotype could be rescued by co-injecting the morpholino with a human PAR3 mRNA (third panel). (b) As a comparison pictures of skin from amotL2a/b morphants AmotL2a (green) and F-actin (red) show similar to what was observed in the par3 morphants, the embryos deficient of AmotL2 showed loss of cytoplasmic actin filaments. Scale bar 10 um. (c) Quantification of fluorescence intensity of AmotL2 at cell junctions in control and par3 MO injected embryos. N (ctrl) = 100 cells, N (par3 MO) = 100 cells. P values ctrl vs par3 MO < 0.0001 as calculated by unpaired t test. (d) Quantification of cell area in control and par3 MO injected embryos. N (ctrl) = 100 cells, N (par3 MO) = 100 cells. P values ctrl vs par3 MO < 0.0001 as calculated by unpaired t test. (e) Quantification of cell geometry in control, par3 morphants and amotl2 as well as embryos co-injected with par3 MO and human PAR3 mRNA. N (ctrl) = 200 cells, N (par3 MO) = 200, N(amotl2 MO) = 200 cells cells, N(par3 MO + hPAR3 mRNA) = 200 cells. p value ctrl vs. par3 MO < 0.0001. p-value par3 MO vs. par3 MO + hPAR3 mRNA < 0.00001, ctrl vs par3 MO + hPAR3 mRNA = 1. Each experiment was repeated at least three times. |
|
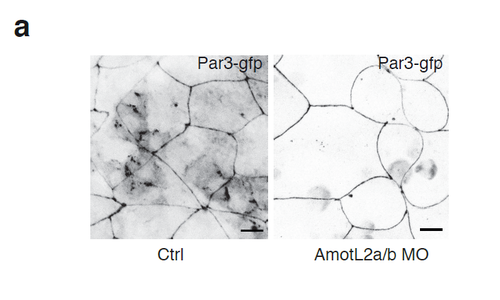
Par3 is efficiently localized to cell-cell junctions independent of AmotL2. (a) Expression of Par3-GFP in control and AmotL2a/b MO treated zebrafish embryos. Par3 show expression at cell-cell junctions independent of AmotL2. Scale bar 10 mm. |
|
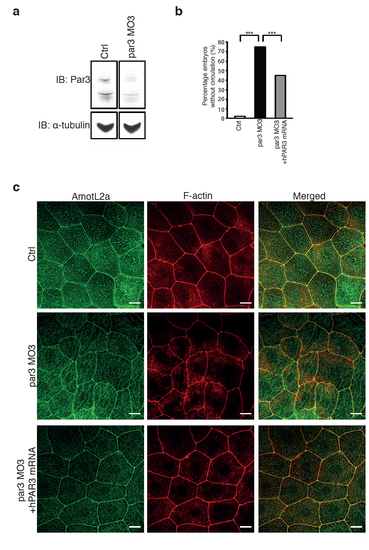
The same phenotypes of the zebrafish vasculature and skin are observed using two different par3 morpholinos. (a) Western blot showing the knock-down efficiency using par3 MO3. α-tubulin was used to control for equal loading. (b) Quantification of the circulation defect in the par3 MO3 embryos. The phenotype could be partially rescued by co-injecting the morpholino with a human PAR3 mRNA. N(ctrl)= 100 embryos, N(par3 MO)= 104 embryos, N(par3 MO+ hPAR3 mRNA)= 124 embryos. *** P≤0.001. (c) Immunofluorescence images showing AmotL2a and F-actin staining of the epidermis of ctrl (top panel), par3 MO3 (mid panel) and par3 MO3 + hPAR3 mRNA (lower panel) injected zebrafish embryos. The structure of the actin cytoskeleton is disrupted in the par3 MO3 injected embryos, correlating with and altered cell shape. Both the cell morphology and the actin filaments could be restored co-injecting the morpholino with a human PAR3 mRNA. PHENOTYPE:
|