- Title
-
Dmrt1 is necessary for male sexual development in zebrafish
- Authors
- Webster, K.A., Schach, U., Ordaz, A., Steinfeld, J.S., Draper, B.W., Siegfried, K.R.
- Source
- Full text @ Dev. Biol.
|
Dmrt1 expression in zebrafish gonads. A-C) Expression of dmrt1 by in situ hybridization on sections of adult gonads. A) In adult ovaries, dmrt1 expression was observed primarily in stage Ib oocytes; staging according to Selman et al. ( Selman et al., 1993). B) Adult testis had dmrt1 expression in nearly all stages of spermatogenesis, except spermatozoa (sz). C) In testis devoid of germ cells (∅ germ line (gl)), dmrt1 was detected primarily in cells lining the testis tubules, indicative of Sertoli cell expression. D-F) Whole mount in situ hybridization on gonads from 5 weeks post fertilization (wpf) fish. D) Dmrt1 was not detected in gonads with ovarian morphology as judged by presence of many early stage oocytes (inset shows an early stage oocyte). E-F) Dmrt1 expression was detected in gonads that morphologically resembled testes (E) and in gonads devoid of germ cells (F). G) RT-PCR on gonadal tissue at 7 wpf, 11 wpf and 28 wpf. At 7 wpf, gonads could not easily be distinguished as ovary or testis during tissue harvesting, therefore, all gonads were mixed at this stage. By 11 wpf, dissected gonads were chosen that resembled either ovaries or testis as judged by morphology during dissection. RT-PCR was also performed on gonads that lacked germ cells (∅ gl) to detect expression in somatic tissues. RT-PCR for the ziwi transcript demonstrated that germ cells were present in intact gonads but absent from germ cell depleted gonads (∅ gl) at all ages (shown for 7 wpf and 11 wpf). Dmrt1 expression was detected by RT-PCR in all samples. Control RT-PCR samples (ctrl) were samples where no reverse-transcriptase was added to the reaction. For 28 wpf samples, two independent samples were run for each: each ovary sample was derived from a single individual while each testes sample contained testes from 4 or more individuals. RT-PCR results for these 28 wpf samples for ziwi and b-actin were previously published ( Siegfried and Nüsslein-Volhard, 2008). scale bars =50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
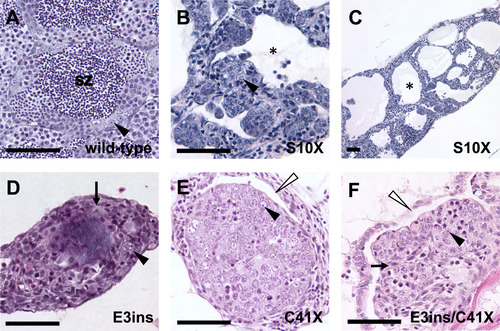
Adult homozygous dmrt1 mutants have disorganized testes with reduced germ cells. A-F) H&E staining of adult male gonads. A) Wild-type adult testes are organized into tubules, with cysts of differentiating germ cells surrounding lumens containing spermatozoa (sz). B and C) Dmrt1S10X mutants form enlarged testis tubules with reduced numbers of germ cells and no spermatozoa. Tubules have large lumens devoid of germ cells (e.g. black asterisk). D) dmrt1E3ins and E) dmrt1C41X mutant testes exhibit similar phenotypes, often displaying increased stroma (e.g. arrows in D, F), with few germ cells and retention of ovarian cavities (white arrowheads). Neither genotype organizes testis tubules nor forms lumens. F) A transheterozygous dmrt1E3ins/C41X mutant looks highly similar to both dmrt1E3insand dmrt1C41Xhomozygotes. Spermatogonia=black arrowhead; lumen filled with spermatozoa=sz; lumen lacking spermatozoa=black asterisk; stroma=arrow; ovarian cavities=white arrowhead. Scale bar=50 µm. PHENOTYPE:
|
|
Dmrt1 is required for normal testis development and gonadal sex-differentiation. A-N) H&E staining of dmrt1E3ins heterozygous and homozygous mutant siblings throughout development. Specimens are progeny from a dmrt1 homozygous female and heterozygous male of from the Pair 1 family of Fig. 3B. These crosses consistently produced progeny with a strong female bias in mutants. A-B) At 3 wpf, mutant gonads typically had fewer apoptotic cells compared to heterozygous siblings (white arrowhead). Immunohistochemistry to detect Cleaved caspase 3 positive cells revealed half the quantity of apoptotic cells in mutant gonads compared to heterozygotes (2.03% vs. 4.04%, respectively, P value <0.0001; N=16 gonads). C-F) 5 wpf heterozygotes progressed to early ovary (C) and testes (D) stages, as indicated by developing oocytes in females (oc), and loss of oocytes combined with lumen formation (asterisk) in males. Mutant gonads were either apparently normal ovaries (E) or testes with no lumens that were typically reduced in size compared to heterozygotes (F). G-J) At 7 wpf heterozygotes were fully committed female (G) and male (H) gonads, whereas mutants that were not clearly female formed either intersex gonads (J) (marked by dying oocytes alongside clustering spermatogonia (arrowhead)), or presumptive testes (not shown). The latter were never observed to form tubules or lumens, and had severely reduced spermatocytes. K-N) By 10 wpf heterozygotes were maturing with ova (K) or spermatids (L). The mutant males (N) were marked by increased stroma (black arrows), and lack of spermatogenesis, although clusters of germ cells were apparent (white arrow). E, I, M) All mutant females developed normally with no detectable phenotype. O) Quantification of the number of gonads in each histological category shown in panels A-N. The number of individuals, “N”, analyzed for each age group is shown in the table. Categories of gonad development observed in each age group reveal delayed transition to testes fate in mutants. Ovary=comprised mainly of oocytes of various stages; transitioning=one or more of the following: apoptotic cells, lumen formation, loss of oocytes, gonial cell clustering; intersex=containing maturing oocytes in addition to testis-like gonial clusters and stroma; testis=containing clusters of spermatogonia (or spermatocytes), having no oocytes, lumen if heterozygous. Apoptotic cells=white arrowhead; spermatogonia=black arrowhead; lumen=black asterisk; oocyte=oc; stroma=black arrow; germ cell cluster=white arrow; liver=L. Scale bar=50 µm. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
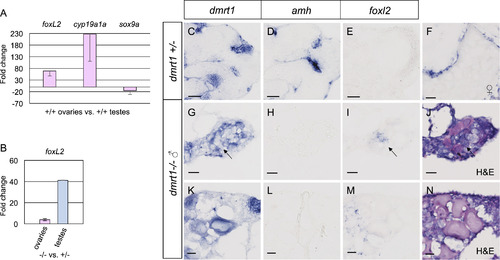
Foxl2 is antagonized by dmrt1 in zebrafish gonads. A-B) Quantitative RT-PCR of adult ovaries and testes from adult fish. A) Comparison of adult wild-type ovaries to testes showed that foxl2 expression was 68 fold higher in ovaries compared to testis, therefore exhibited ovary-enriched expression. The expected upregulation of the granulosa cell marker, cyp19a1a, and diminished expression of the Sertoli cell marker sox9a were also observed in these samples. B) Comparison of adult dmrt1E3ins mutant ovaries to wild-type ovaries (left) and mutant testes to wild-type testes (right) revealed a 4-fold increase in foxl2 expression in ovaries and a 41-fold increase in foxl2 in testes in dmrt1 mutants compared to wild-type. C-N) In situ hybridization on adult dmrt1C41X +/− and −/− gonad cryosections. C-E are serial sections of the same testis. G-I are serial sections of the same gonad and J is an H&E stain of panel I. K-M are serial sections of the same gonad and N is an H&E stain of M. Headings designate the probe used. C-E) Dmrt1 heterozygous testes showed expected localization of dmrt1 (C) and amh (D) in germ cells and Sertoli cells, respectively, whereas foxl2 (E) was not detected. F) In wild-type ovaries, foxl2 transcript localized to the somatic cells of pre-vitellogenic and vitellogenic oocytes, a vitellogenic oocyte is shown. G-N) Two dmrt1 male mutant gonads are shown. G-J) A testis-like gonad with scant residual oocytes. K-N) An intersex gonad containing pre-vitellogenic oocytes. G, K) Dmrt1 was expressed in residual oocytes, but was not detected in all germ cells (arrow). H, L) Amh was not detected. I, M) Misexpression of foxl2 was found interstitially, in presumptive gonial cells but not in the follicles, as supported by H&E staining (J, N). Detection of dmrt1 transcript in homozygous mutants is attributed to the dmrt1C41X allele producing mRNA. Scale bars=20 µm. |
|
Expression of foxl2 in comparison to amh in wild-type adult ovaries, as detected by in situ hybridization. Panels show adjacent sections of the same ovary. Expression of these transcripts was detected in the somatic cells surrounding oocytes from stage 1B (arrowhead) through late stage II/early stage III. Both probes nonspecifically stained the yolk vesicles in late stage III oocytes. Scale bar = 50 microns. |
Reprinted from Developmental Biology, 422(1), Webster, K.A., Schach, U., Ordaz, A., Steinfeld, J.S., Draper, B.W., Siegfried, K.R., Dmrt1 is necessary for male sexual development in zebrafish, 33-46, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.