- Title
-
IGFBP1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation
- Authors
- Lu, J., Liu, K.C., Schulz, N., Karampelias, C., Charbord, J., Hilding, A., Rautio, L., Bertolino, P., Östenson, C.G., Brismar, K., Andersson, O.
- Source
- Full text @ EMBO J.
|
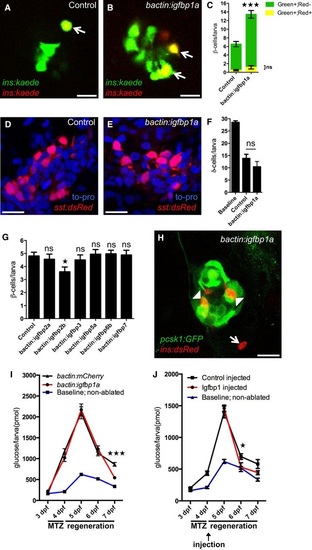
Genetic screening in zebrafish identifies igfbp1a as a promoter of βcell regeneration A. Schema of the analysis of gene expression in islets isolated from control larvae and larvae subjected to βcell ablation. β cells were ablated by exposing nitroreductase (NTR)expressing transgenic larvae to metronidazole (MTZ) from 3 to 4 dpf. Islets were then isolated, and their RNA extracted and analyzed by microarray. Out of the 470 genes that were upregulated more than 50%, 33 genes encoded proteins that harbored a signal peptide for secretion (according to the algorithm of SignalP). Excluding genes that encode enzymes or proteins with a transmembrane (TM) domain, we selected 11 genes for overexpression studies in zebrafish larvae (C-E). B. Microarray heat map showing the upregulation in expression of the 11 candidate genes in βcellablated versus control islets. Igfbp1a and b were the genes whose expression increased the most after βcell ablation. C. Schema of the construct used for overexpression of the candidate genes (under the control of the betaactin promoter), and expression of GFP in the heart (as an internal control for genomic integration).D, E. Representative images at 6 dpf of Tg(ins:kaede);Tg(ins:CFPNTR) transgenic larvae that had been injected at the 1-2 cell stage with transposase mRNA (control) or transposase mRNA + bactin:igfbp1a (bactin:igfbp1a), subjected to βcell ablation by metronidazole (MTZ) during 3-4 dpf, and subsequently allowed to regenerate for 2 days. The GFP+ heart (arrowhead) visualizes successful integration of the construct. Islets are indicated by white arrows. Scale bars: 100 µm. F. Quantification of βcell regeneration at 6 dpf in control (n = 23), bactin:igfbp1aoverexpressing (n = 13), and bactin:igfbp1boverexpressing (n = 8) Tg(ins:kaede);Tg(ins:CFPNTR) larvae; ***P = 0.0002, ns = nonsignificant (P = 0.3106). G. Immunohistochemistry showing Igfbp1 protein expression in 6 dpf Tg(ins:GFP) following βcell ablation between 3 and 4 dpf. Scale bar: 50 µm.H. Both liverspecific (lfabp promoter; n = 46) and widespread (bactin promoter; n = 18) overexpression of igfbp1a increase βcell regeneration when compared to control (n = 77); ***P < 0.001. I-M. Quantification of β cells with or without βcell ablation and igfbp1a overexpression by confocal microscopy, which detects even weakly insulinexpressing β cells; ****P < 0.0001. Scale bars: 15 µm. |
|
Igfbp1a′s effect on β-cell regeneration is specific and functionally relevant A-C. Igfbp1a promotes βcell regeneration, rather than βcell survival. To trace β cells, we exposed Tg(ins:kaede) larvae to UV light (which causes the existing Kaede protein to switch from emitting green fluorescence to emitting red fluorescence) just before ablating the β cells by MTZ treatment from 3 to 4 dpf. After regeneration, the newly formed β cells are green, whereas the β cells that survived ablation are yellow (overlap of green and red). Representative confocal images (A, B) at 6 dpf of control and bactin:igfbp1a-overexpressing Tg(ins:kaede);Tg(ins:CFPNTR) transgenic larvae; arrows indicate surviving (yellow) β cells. Scale bars: 10 µm. (C) Quantification of βcell regeneration (green bars) and βcell survival (yellow bars) per larva at 6 dpf. ***P < 0.001; n = 20 larvae in the control group, n = 27 larvae in the bactin:igfbp1a-overexpressing group. D-F. Igfbp1a does not promote δcell regeneration. We treated control and bactin:igfbp1a-overexpressing Tg(sst:flagNTR);Tg(sst:dsRed) larvae with MTZ from 3 to 4 dpf to ablate δ cells, and then allowed them to regenerate until 6 dpf. Representative confocal images (D, E) at 6 dpf of control and bactin:igfbp1a-overexpressing larvae showing comparable number of δ cells after 2 days of regeneration. Scale bars: 15 µm. (F) Quantification of the total number of δ cells per δcellablated larva at 6 dpf compared to the baseline number of δ cells in nonablated control larvae. ns, P = 0.2325; n = 13 in the control group, n = 7 in the bactin:igfbp1a group. G. Other Igfbps do not promote βcell regeneration. We injected Tg(ins:kaede);Tg(ins:CFPNTR) transgenics with either transposase mRNA (control; n = 31) or transposase mRNA + one of six different igfbps, igfbp2a (n = 25), igfbp2b (n = 25), igfbp3 (n = 26), igfbp5a (n = 17), igfbp6b (n = 34), and igfbp7 (n = 17), treated them with MTZ from 3 to 4 dpf to ablate their β cells, and then quantified their β cells after 2 days of regeneration, at 6 dpf; ns=nonsignificant (igfbp2a P = 0.986, igfbp3 P = 0.979, igfbp5a P = 0.999, igfbp6b P = 0.997, and igfbp7 P = 0.999); igfbp2b *P = 0.037. H. Tg(pcsk1:GFP) is expressed in regenerating β cells within the islet (arrowheads), but not outside the islet (arrow), at 6 dpf in larvae overexpressing igfbp1a. Scale bar: 15 µm. I, J. Freeglucose levels during βcell regeneration in control (bactin:mCherry) and bactin:igfbp1a-overexpressing Tg(ins:kaede);Tg(ins:CFPNTR) larvae (I), as well as in Tg(ins:kaede);Tg(ins:CFPNTR) larvae injected in the pericardial sac at 4 dpf with 1 ng of recombinant mouse Igfbp1 (J). We treated larvae with MTZ from 3 to 4 dpf to ablate their β cells and monitored their freeglucose levels at 3-7 dpf. Freeglucose levels were significantly lower after genetic igfbp1a overexpression or Igfbp1protein injection (red lines) than in controls (black lines) at 7 and 6 dpf, respectively. Baseline reference levels of free glucose throughout development are shown for a different set of larvae without βcell ablation. n = 24 larvae (four pools of six larvae) per data point; ***P < 0.001, *P < 0.05. EXPRESSION / LABELING:
|
|
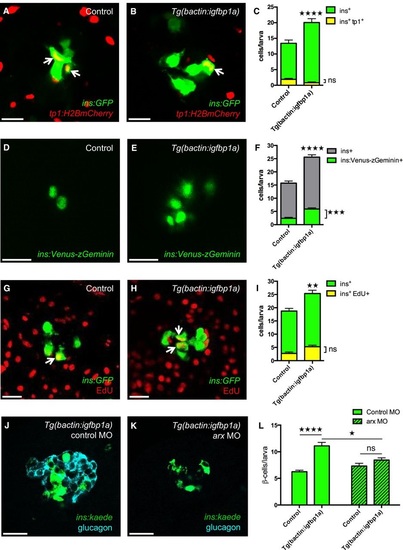
Cellular mechanisms of Igfbp1a′s effect on βcell regeneration A-C. Tg(ins:GFP);Tg(tp1:H2BmCherry);Tg(ins:FlagNTR) transgenics, with or without Tg(bactin:igfbp1a), were treated with MTZ from 3 to 4 dpf to ablate the β cells and were then allowed to regenerate from 4 to 6 dpf. Representative confocal images at 6 dpf of control (A) and Tg(bactin:igfbp1a) (B) larvae showing a modest number of ins+ tp1+ coexpressing cells, indicated by arrows, after 2 days of regeneration. Scale bars: 15 µm. (C) Quantification of the total number of β cells (green bars) at 6 dpf, and of β cells expressing Tg(tp1:H2BmCherry), that is, of ductal origin (yellow bars). ****P < 0.0001, ns= nonsignificant. n = 23 larvae in the control group, n = 17 larvae in the Tg(bactin:igfbp1a) group. D-F. Tg(ins:VenuszGeminin) was examined in control and Tg(bactin:igfbp1a) larvae at 6 dpf, after βcell ablation 3-4 dpf by using Tg(ins:FlagNTR). Representative confocal images (D, E) at 6 dpf of control and Tg(bactin:igfbp1a) larvae showing cell cycle activation of β cells in green. Scale bars: 15 µm. (F) Quantification of the total number of β cells with activated cell cycle at 6 dpf, ***P < 0.001, n = 32 larvae in the control group, n = 41 larvae in the Tg(bactin:igfbp1a) group. The number of β cells with activated cell cycle is displayed together with the total number of β cells in experiments with the same setup, ****P < 0.0001. n = 39 larvae in the control group, n = 33 larvae in the Tg(bactin:igfbp1a) group. G-I. EdU was used as a marker for cell cycle progression. Tg(ins:H2BGFP);Tg(ins:FlagNTR) transgenics, with or without Tg(bactin:igfbp1a), were treated with MTZ from 3 to 4 dpf to ablate their β cells, and subsequently incubated with EdU during regeneration from 4 to 6 dpf. Representative confocal images (G, H) at 6 dpf of control and Tg(bactin:igfbp1a) larvae showing β cells in green and the β cells that had incorporated EdU in yellow (green and red overlap; arrowheads). Scale bars: 20 µm. (I) Quantification of the total number of β cells (green bars) at 6 dpf and of β cells that incorporated EdU (yellow bars) during βcell regeneration from 4 to 6 dpf. **P < 0.01, ns = nonsignificant. n = 16 in both the control and the Tg(bactin:igfbp1a) group.J-L. Tg(ins:kaede);Tg(ins:CFPNTR) transgenics, with or without Tg(bactin:igfbp1a), were injected with a control morpholino or a morpholino that knocked down arx, which is necessary for αcell differentiation and thus glucagon expression (J, K). Scale bars: 20 µm. (L) Quantification of the total number of regenerating β cells at 4 dpf (after βcell ablation at 2-3 dpf). ****P < 0.0001, *P < 0.05, ns=nonsignificant. n = 42, 20, 48 and 20, respectively. |
|
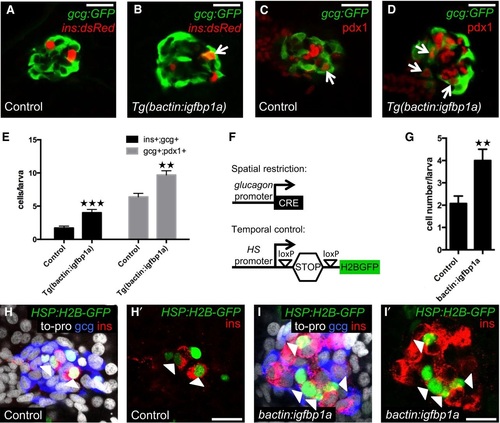
Igfbp1a increases βcell regeneration by potentiating α to βcell transdifferentiation A-E. Control and Tg(bactin:igfbp1a)overexpressing larvae were treated with MTZ from 3 to 4 dpf to ablate β cells and analyzed at 6 dpf, after 2 days of regeneration. Representative confocal images (A, B) of control and Tg(bactin:igfbp1a)overexpressing Tg(ins:dsRed);Tg(gcg:GFP);Tg(ins:FlagNTR) larvae. A bihormonal glucagon and insulinexpressing cell (gcg+;ins+) is indicated by an arrow. Representative confocal images (C, D) of control and Tg(bactin:igfbp1a)overexpressing Tg(gcg:GFP);Tg(ins:FlagNTR) larvae stained for Pdx1. Pdx1 and glucagonexpressing cells (pdx1+;gcg+) are indicated by arrows. Scale bars: 20 µm. (E) Quantification of gcg+;ins+ and pdx1+;gcg+ cells in the control and Tg(bactin:igfbp1a) group; ***P < 0.001, **P = 0.0012; n = 18 and 18 in the control groups, n = 15 and 10 in the Tg(bactin:igfbp1a) groups, for the gcg+;ins+ and pdx1+;gcg+ quantification, respectively. F-I. Lineagetracing evidence supports α to βcell transdifferentiation. (F) Schema for temporal and spatial lineage tracing. loxPmediated excision of the STOP cassette permits heatinducible expression of the stable fusionprotein H2BGFP. Control and bactin:igfbp1aoverexpressing Tg(hsp:loxPmCherrySTOPloxPH2BGFP);Tg(gcg:CRE);Tg(ins:FlagNTR) larvae were first heatshocked at 3 dpf to label the glucagonexpressing cells with the stable fluorescent protein H2BGFP, thereafter treated with MTZ from 3 to 4 dpf to ablate the β cells, and analyzed at 6 dpf after 2 days of regeneration. Note that the temporal control of the lineage tracing is mediated by a heatshock. (G) Quantification of insulinexpressing cells that originate from α cells. Control larvae, n = 13; bactin:igfbp1a larvae, n = 16. **P = 0.0051. Representative confocal images (H, I) at 6 dpf (after 2 days of regeneration) of control and bactin:igfbp1aoverexpressing larvae stained for insulin, glucagon, topro, and H2BGFP. ins+;H2BGFP+ cells are indicated by the arrowheads, and H′ and I′ show staining against only insulin and H2BGFP. Moreover, most ins+;H2BGFP+ cells no longer express glucagon. Scale bars: 15 µm. |
|
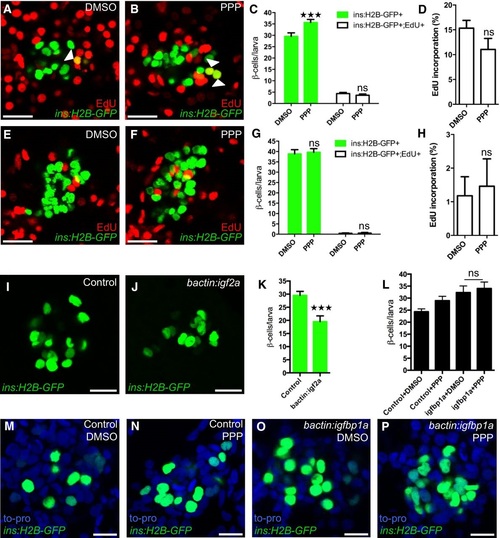
Inhibition of the Igf pathway mimics Igfbp1a′s stimulatory effect on βcell regeneration A-H. PPP, an IGF1R inhibitor, promotes βcell regeneration. Tg(ins:H2BGFP);Tg(ins:FlagNTR) larvae were treated with MTZ from 3 to 4 dpf to ablate the β cells and then treated with EdU and DMSO or with EdU and PPP during regeneration from 4 to 6 dpf. Representative confocal images (A, B) at 6 dpf of DMSO and PPPtreated larvae displaying β cells in green and the β cells that had incorporated EdU as yellow overlap (arrowheads). (C) Quantification of the total number of β cells (green bars) at 6 dpf, and β cells that had incorporated EdU (white bars) from 4 to 6 dpf during βcell regeneration. ***P = 0.0003, P = 0.8607, respectively. (D) Rate of βcell proliferation displayed as the percentage of β cells that incorporated EdU. P = 0.1194. n = 18 larvae for the DMSOtreated group, n = 17 larvae for the PPPtreated group. (E-H) To examine whether PPP affected βcell proliferation during regular development, Tg(ins:H2BGFP) larvae were treated with EdU and DMSO or PPP from 4 to 6 dpf. Representative confocal images (E, F) of 6 dpf DMSO and PPPtreated larvae displaying β cells in green and the β cells that had incorporated EdU as yellow overlap. Scale bars: 20 µm. (G) Quantification of the total number of β cells (green bars) and β cells that had incorporated EdU (white bars) per larva from 4 to 6 dpf. P = 0.9098 and 0.9976, respectively. (H) Rate of βcell proliferation displayed as the percentage of β cells that incorporated EdU. P = 0.7822. n = 16 larvae for DMSOtreated group, 18 larvae for PPPtreated group. I-K. Activation of the Igf pathway reduces βcell regeneration. Control and bactin:igf2aoverexpressing Tg(ins:H2BGFP);Tg(ins:FlagNTR) larvae were treated with MTZ from 3 to 4 dpf to ablate β cells and subsequently let to regenerate from 4 to 6 dpf. Representative confocal images (I, J) of 6 dpf control and bactin:igf2aoverexpressing larva displaying β cells in green. Scale bars: 15 µm. (K) Quantification of the total number of β cells per larva at 6 dpf following βcell regeneration from 4 to 6 dpf. ***P < 0.0001. n = 28 larvae for control, n = 15 larvae for bactin:igf2a. L-P. No synergistic effect was observed for igfbp1a and PPP. Control and bactin:igfbp1aoverexpressing Tg(ins:H2BGFP);Tg(ins:FlagNTR) larvae were treated with MTZ from 3 to 4 dpf to ablate β cells and subsequently treated with DMSO or PPP during regeneration from 4 to 6 dpf. (L) Quantification of the total number of β cells per larva. n > 10 (n = 23, 20, 14, 13). P = 0.9546. Representative confocal images (M-P) of 6 dpf control or bactin:igfbp1a overexpressing larvae treated with either DMSO or PPP, displaying β cells after 2 days regeneration. Scale bars: 10 µm. EXPRESSION / LABELING:
|
|
Igfbp1a also increases βcell regeneration in 1 month old zebrafish A, B. Representative confocal projections of the whole pancreas (dashed lines) of 35dayold Tg(ins:H2BGFP);Tg(ins:FlagNTR) transgenics, with or without Tg(bactin:igfbp1a), that were subjected to βcell ablation between day 30 and 31 and then allowed to regenerate for 4 days. Scale bars represent 100 µm. C. Body length of control and Tg(bactin:igfbp1a) zebrafish; ****P < 0.0001. D-H. Quantification of βcell regeneration was automated with an ImageJ script. (D) Insulinpositive area per zebrafish. (E) Relative insulinpositive area per body length; **P < 0.01. (F) The percentage of the pancreas area that was insulinpositive was significantly larger in Tg(bactin:igfbp1a) than in controls; ****P < 0.0001. (G) The total number of recorded units of adjacent ins:H2BGFP+ pixels (units ranging from single β cells to βcell clusters) did not differ between control and Tg(bactin:igfbp1a) zebrafish. (H) The size of recorded ins:H2BGFP+ units was on average larger in Tg(bactin:igfbp1a) than in controls; *P = 0.039. n = 26 in the control group, n = 27 in the Tg(bactin:igfbp1a) group. EXPRESSION / LABELING:
PHENOTYPE:
|
|
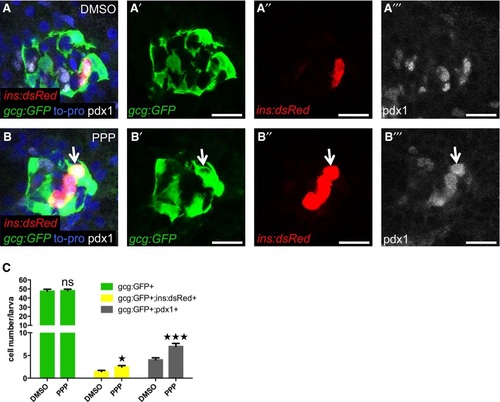
The IGF1R inhibitor PPP increases βcell regeneration and coexpression of insulin and pdx1 with glucagon A-C. Pdx1 expression in 6dpf larvae during regeneration. Tg(ins:dsRed);Tg(gcg:GFP);Tg(ins:FlagNTR) larvae were treated with MTZ from 3 to 4 dpf to ablate the ² cells and subsequently treated with DMSO or the IGF1R inhibitor PPP from 4 to 6 dpf. Representative confocal images (A, B) at 6 dpf of DMSO or PPPtreated larvae. A gcg+ins+pdx1+ cell is indicated by an arrow. Scale bars: 15 µm. (C) Quantification of gcg+, gcg+ins+, and pdx1+gcg+ cells (P = 0.8409, *P = 0.0232, and ***P = 0.0007, respectively) in DMSO and PPPtreated groups. n = 20 for each group. Results are presented as mean values ± SEM and analyzed with ANOVA. Related to Fig 4. |
|
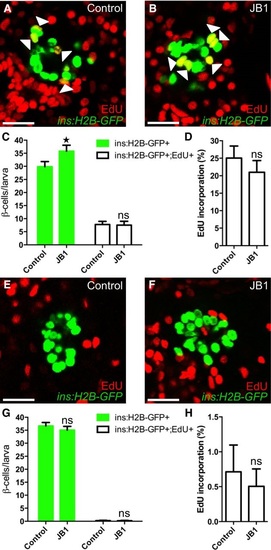
The IGF1R inhibitor JB1 promotes βcell regeneration A-D. Tg(ins:H2BGFP);Tg(ins:FlagNTR) larvae were treated with MTZ from 3 to 4 dpf to ablate the β cells. DMSO or JB1 was then injected into the larval pericardial cavity at 4 dpf, and the larvae were treated with EdU from 4 to 6 dpf. Representative confocal images (A, B) at 6 dpf of DMSO and JB1injected larvae, showing β cells in green and the β cells that incorporated EdU as yellow (green and red overlap; see arrowheads). Scale bars: 20 µm. (C) Quantification of the number of all β cells (green bars) and of β cells that incorporated EdU (white bars) per larva at 6 dpf. *P = 0.0422; ns=nonsignificant (P = 0.9944). (D) Rate of βcell proliferation, shown as the percentage of β cells that incorporated EdU. ns=nonsignificant (P = 0.5885). n = 12 larvae in the DMSOinjected group; n = 19 larvae in the JB1injected group. E-H. To determine whether inhibition of Igf signaling affects βcell proliferation during regular development, we treated DMSO or JB1injected Tg(ins:H2BGFP) larvae with EdU in the absence of βcell ablation. DMSO or JB1 was injected into pericardial cavity of Tg(ins:H2BGFP) larvae at 4 dpf, and the larvae were then treated with EdU from 4 to 6 dpf. Representative confocal images (E, F) at 6 dpf of DMSO and JB1injected larvae, showing β cells in green and the cells that incorporated EdU in red. Scale bars: 20 µm. (G) Quantification of the number of all β cells (green bars) and of β cells that incorporated EdU (white bars) from 4 to 6 dpf. ns=nonsignificant (P = 0.4088 and 0.9997, respectively). (H) Rate of βcell proliferation, shown as the percentage of β cells that incorporated EdU. P = 0.8504. n = 20 larvae in the DMSOinjected group, n = 27 larvae in the JB1injected group. |