- Title
-
DNA hypomethylation induces a DNA replication-associated cell cycle arrest to block hepatic outgrowth in uhrf1 mutant zebrafish embryos
- Authors
- Jacob, V., Chernyavskaya, Y., Chen, X., Tan, P.S., Kent, B., Hoshida, Y., Sadler, K.C.
- Source
- Full text @ Development
|
Reduction in uhrf1 mRNA expression precedes onset of mutant phenotype. (A) Cumulative survival of uhrf1 mutant larva and wild-type siblings. (B) uhrf1 mRNA levels were measured by qPCR over a period of 25-200 hours post fertilization (hpf). Embryos were individually genotyped prior to 72h to identify mutants and older larvae were sorted based on morphological phenotype. Data are average fold change in expression from three or more clutches. *P<0.05 by Student′s t-test. (C) The same wild-type and uhrf1 mutant embryo was imaged every day, except at 216hpf, when larvae from a separate clutch were used. Labels on the head, jaw, eye, liver (highlighted using the fabp10:dsRed transgene) and gut indicate the first time point at which defects in these tissues become visible in uhrf1 mutants. (D) Left liver lobe area was measured in four wild-type and three mutant larvae at 56-120hpf. The relative size of the uhrf1 mutant liver relative to wild-type siblings is labeled in red. *P<0.05 by Student′s t-test, error bars are s.d. PHENOTYPE:
|
|
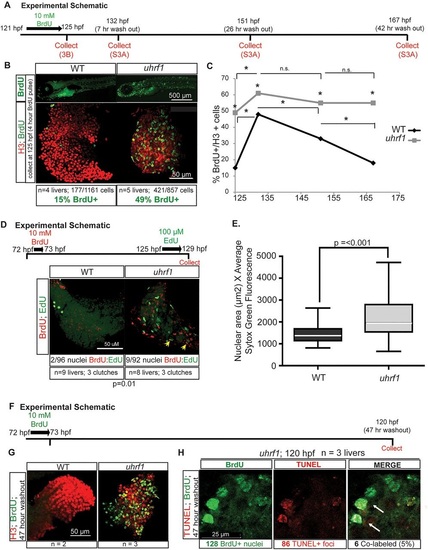
uhrf1 mutant hepatocytes apoptose after failing to proceed through S phase. (A) BrdU pulse-chase experiment overview. The figures and figure panels containing the data from each collection point are indicated. (B) Immunofluorescence for BrdU and H3 to label all nuclei on livers from uhrf1 and wild-type larvae collected at 124hpf, immediately after BrdU pulse. BrdU- and H3-positive cells were counted for four or five livers per genotype. (C) Average number of BrdU-positive nuclei in three to five livers per sample relative to nuclear H3 (see panel B and supplementary material Fig. S3D for raw numbers). Asterisks on the gray line indicate P<0.05 when comparing mutant and wild-type values; asterisks or n.s. (not significant) above the bars indicate differences between samples of the same genotype at different times. (D) Schematic of BrdU pulse at 72hpf chased with EdU at 125hpf. Livers were processed for both nucleotide analogs to determine percent of co-labeled nuclei. (E) Relative DNA content in uhrf1 compared with wild-type hepatocytes at 125hpf, as measured by Sytox Green staining. Top and bottom of box plot designate 75% and 25% of the population, respectively, separated by the median; bars represent 10th and 90th percentiles. (F) BrdU pulse-chase experiment outline. (G) Embryos treated for 1h with 10mM BrdU at 72hpf were collected at 120hpf (47h post-washout) and stained for H3 and BrdU. (H) Hepatocyte nuclei with both BrdU and TUNEL staining are labeled with white arrows. |
|
Cell cycle arrest is an acute response to the loss of uhrf1. (A) Acridine Orange staining of whole fish was imaged at 56 and 72hpf. Averaged intensity of fluorescence from the head, jaw and ear of three or four larvae (n) is indicated with the s.d. Arrows point to the regions with enhanced cell death in mutant larvae. (B) Whole fish were pulsed with BrdU at 56hpf for 1h and processed for BrdU immunofluorescence at 72hpf. Arrow points to intense labeling in the liver. (C) Tg(hsp70I:UHRF1-GFP; fabp10:ds-Red);uhrf1 and wild-type siblings were heat-shocked to induce expression of UHRF1 at the indicated times. The liver is outlined in red. (D) The left liver lobe area was quantified in three or four fish per time point. All fish contain the Tg(hsp70l:UHRF1-GFP) transgene. Heat shock had no effect on liver size in WT fish. Box plots are as described in Fig. 3E. *P<0.05 by Student′s t-test. The percent difference in the area of the left liver lobe of the mutants relative to wild-type is indicated. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Uhrf1 acts cell-autonomously to regulate liver outgrowth. (A) Wild-type and uhrf1 5dpf larvae with or without the Tg(fabp10:UHRF1-EGFP)Low transgene. The liver is outlined. (B) The area of the left liver lobe was quantified in 10-16 larvae from three clutches of each genotype (left panel). Top and bottom of box plot designate 75% and 25% of the population, respectively, separated by the median; bars represent 10th and 90th percentiles. *P<0.05. The percent relative to wild-type liver is indicated. (C) qPCR on cDNA from dissected livers. Student′s t-test was used to determine significance between ΔΔCt values of uhrf1 mutants with and without the Tg(fabp10:UHRF1-EGFP)Low transgene and percentage of upregulation in mutants with and without the transgene is indicated. (D) Representative image of more than two TUNEL-stained livers from each sample with the average number of TUNEL (+) foci for each sample indicated in red. Livers without the UHRF1-EGFP expression were stained with Cy5-SA to highlight hepatocytes. (E) Cumulative survival of one cohort of fish. The number of animals in each sample is indicated. Log rank test was used to calculate P-value. |
|
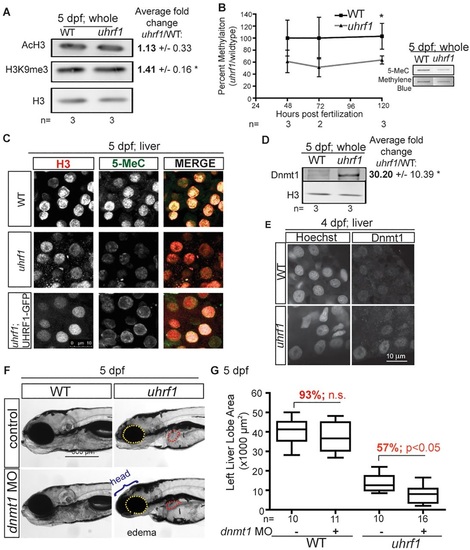
The uhrf1 mutant phenotype is mediated by hypomethylation. (A) Western blot of AcH3 and H3K9me3 levels in whole 5dpf larvae normalized to H3. Average ratios to H3 from three clutches are indicated with the s.d. *P<0.05 by Student′s t-test. (B) 5-MeC levels in uhrf1 mutant embryos and wild-type siblings, as measured by slot blot from at least three clutches. Mutant embryos were identified by genotyping at 48-72hpf and sorted based on morphological criteria at 96-120hpf. (C) 5-MeC immunofluorescence on livers of 5dpf wild type, uhrf1 mutants and Tg(fabp10:UHRF1-EGFP)Low;uhrf1 mutants. (D) Western blot of Dnmt1 in whole embryos. Average ratio of Dnmt1 to H3 in three clutches is indicated with the s.d. *P<0.05 by Student′s t-test. (E) Dnmt1 immunofluorescence and Hoechst staining for DNA in livers of 4dpf larvae. (F) Enhancement of the phenotypes in uhrf1 mutants injected with dnmt1 morpholino are indicated. (G) Quantification of left liver lobe size in 10-17 larvae on 5dpf. Top and bottom of box plot designate 75% and 25% of the population, respectively, separated by the median; bars represent 10th and 90th percentiles. *P<0.05. The percentage of the average liver size in dnmt1 morphants compared with the control injected larvae is indicated in red. n.s., not significant. |
|
The cell cycle phenotype in uhrf1 mutants is phenocopied by dnmt1 mutation. (A) Average levels of 5-MeC in 5dpf whole dnmt1 embryos and wild-type siblings was measured by slot blot in pools of embryos from three clutches. (B) qPCR of cell cycle genes from 5dpf whole larvae (black) and dissected livers (gray) from three to five clutches of dnmt1 mutants and wild-type siblings. Student′s t-test on ΔΔCt values was used to determine significance; *P<0.05. (C) Western blot of Mcm3, pH3 and PCNA in 5dpf whole dnmt1 wild-type and mutant fish with the average fold change of the ratio to H3 indicated for three samples ±s.d. There was no Mcm3 detected in wild-type larvae, precluding calculation of the fold change. (D) Representative image of BrdU and H3 immunoflourescence on a liver from dnmt1 and wild-type larvae exposed to BrdU from 120 to 125hpf. The average ratio of BrdU/H3-positive nuclei is indicated. |