- Title
-
Selective Small Molecule Targeting β-Catenin Function Discovered by In Vivo Chemical Genetic Screen
- Authors
- Hao, J., Ao, A., Zhou, L., Murphy, C.K., Frist, A.Y., Keel, J.J., Thorne, C.A., Kim, K., Lee, E., and Hong, C.C.
- Source
- Full text @ Cell Rep.
|
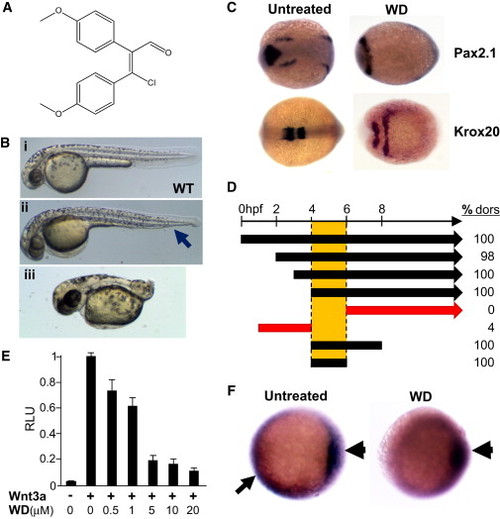
WD, which Dorsalizes Zebrafish Embryo, Inhibits Canonical Wnt Signaling (A) Chemical structure of WD, (Z) 3-chloro-2,3-bis(4-methoxyphenyl)acrylaldehyde, is shown. (B) WD dorsalizes zebrafish embryos. (i) Vehicle-treated WT zebrafish embryo at 48 hpf. (ii and iii) Dorsalized embryos treated with 10 μM WD. (i) Mildly dorsalized embryo is characterized by loss of ventral tail fin (arrow), whereas (ii) severely dorsalized embryo is characterized by severely shortened tail. (C) In situ hybridization of six-somite-stage (12 hpf) embryos was performed to evaluate expression of the dorsal markers Pax2.1, which marks the midhindbrain boundary, and Krox20, which marks rhombomeres 3 and 5. In comparison to untreated embryos (left), Pax2.1 and Krox20 expression are dramatically expanded in WD-treated embryos (right). Dorsal view, anterior is to the left. (D) Time window for dorsalization by WD (20 μM) is between 4 and 6 hpf. Numbers shown on right are percentage (%) of embryos dorsalized (dors) upon various WD treatment schemes. Left end of each bar or arrow represents the time when WD treatment was initiated, and the right end of each bar represents the time when WD was washed out (n > 100 embryos for each condition). (E) WD inhibited Wnt3-induced signaling in β-catenin-responsive TOPFLASH reporter assay (n = 4; results are represented as mean RLU [relative luciferase units] ± SE). (F) In situ hybridization for GFP expression in Wnt-dependent TOPFLASH-GFP transgenic zebrafish reveals that WD selectively abrogated Wnt signaling in the ventral and lateral regions (arrow) of the 50% epiboly stage (5.3 hpf) embryos, but not within the dorsal organizer (arrowhead). Ventral side is to the left. See also Figures S1, S2, and S3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
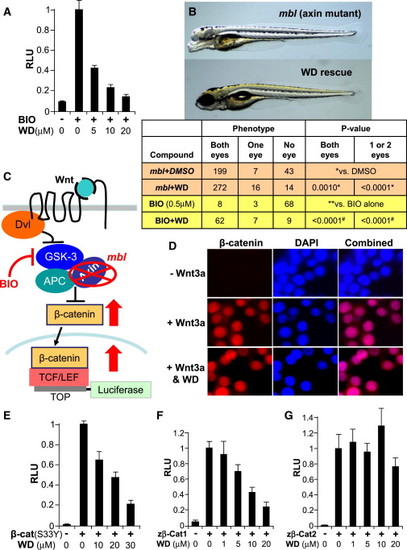
WD Blocks Wnt Signaling Downstream of β-Catenin Destruction Complex (A) WD inhibited Wnt signaling induced by BIO in TOPFLASH-luciferase assays in STF293 cells (n = 4; results are represented as mean RLU ± SE). (B) WD (20 μM) rescued the loss of telencephalon and eyes in mbl mutant zebrafish with defective Axin1 gene. Top view shows untreated Axin1/mbl mutant zebrafish embryo (3 days old). Bottom view presents WD-treated embryo with normal/restored eyes and telencephalon. Quantification of telencephalon/eye loss phenotype in mbl mutants and BIO-treated embryos, following treatment with WD or DMSO control, is shown. p value was determined using Student’s two-tailed t test. (C) Model illustrates TOPFLASH-luciferase assay, with components of the Wnt/β-catenin signaling and various means to perturb them. Briefly, disruption of the β-catenin degradation complex, either through pharmacological inhibition of GSK3β using the small molecule BIO or a genetic mutation in Axin (mlb), results in nuclear β-catenin accumulation and subsequent Wnt reporter activation. (D) WD treatment does not block Wnt3a-induced β-catenin nuclear translocation in RKO cells. RKO cells were immunostained for β-catenin (red) and counterstained with DAPI (blue) following 24 hr incubation without Wnt3a, with Wnt3a, and with Wnt3a plus WD (20 μM). (E) WD inhibited Wnt signaling induced by overexpression of the constitutively active human β-catenin (cat) mutant (S33Y) in TOPFLASH-luciferase assay (n = 4; results are represented as mean RLU ± SE). (F) WD blocked Wnt signaling induced by overexpression of the zebrafish β-catenin-1 in TOPFLASH-luciferase assay in STF293 cells (n = 3). (G) WD did not block Wnt signaling induced by overexpression of the zebrafish β-catenin-2 (n = 3) in STF293 cells. See also Figure S4. PHENOTYPE:
|