- Title
-
Compensatory Role of Inositol 5-Phosphatase INPP5B to OCRL in Primary Cilia Formation in Oculocerebrorenal Syndrome of Lowe
- Authors
- Luo, N., Kumar, A., Conwell, M., Weinreb, R.N., Anderson, R., and Sun, Y.
- Source
- Full text @ PLoS One
|
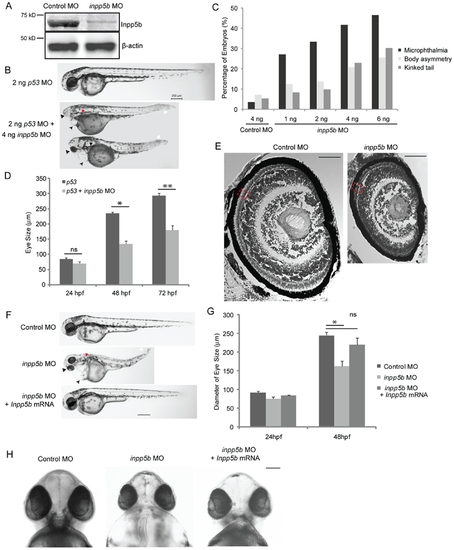
Inpp5b morpholino affects multiple organ development in zebrafish. (A) Immunoblot analysis of 40 microgram of total lysates of zebrafish embryo injected with control MO (4 ng) or inpp5b MO (4 ng) at 48 hpf with anti-INPP5B and anti-beta-actin antibodies. (B) Zebrafish embryos were injected with p53 MO (2 ng) or p53 MO (2 ng) and inpp5b MO (4 ng). Representative phenotypes of microphthalmia (black arrowhead), pericardial edema (small arrow), body axis asymmetry, kinked tail (white arrow), pronephric cyst formation (red arrow), and hypopigmentation were observed at 48 hpf. Scale bar 250 micron. (C) Dose-dependent effect of morpholinos in zebrafish. Control or inpp5b MO at indicated doses was injected into zebrafish embryos, and phenotypes of microphthalmia, kinked tail, and body asymmetry were quantified at 48 hpf (ANOVA, F = 92, p = 3.6E-10), kinked tail (ANOVA, F = 3.6, p = 0.08), and body asymmetry (ANOVA, F = 5.2, p = 0.04). (N = the number of injected embryos N >50). (D) Quantification of eye size of morphants at 24 hpf, 48 hpf and 72 hpf. The eye size was determined by the longest diameters in dorsal view. (N >40 embryos, three independent experiments, unpaired t-test, * p = 4.98E-07 ** p = 2.1E-10, ns, not statistically significant). (E) Cresyl violet staining of ocular sections of zebrafish larvae (5 dpf) injected with control MO (4 ng) or inpp5b MO (4 ng). Scale bar 30 micron. (F) Zebrafish embryos were injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and Inpp5b WT mRNA (Inpp5b mRNA, 500 pg). Representative phenotypes of microphthalmia (arrowhead), pericardial edema (arrow), body axis asymmetry, kinked tail (white arrow), pronephric cyst formation (red arrow), and hypopigmentation were observed at 48 hpf. Scale bar 250 micron. (G) Quantification of eye size of zebrafish morphants at 24 hpf and 48 hpf. (N >40 embryos, three independent experiments, unpaired t-test, * p = 4.5E-05). (H) The ventral sides of embryos were injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and Inpp5b WT mRNA (500 pg). Scale bar 100 micron. |
|
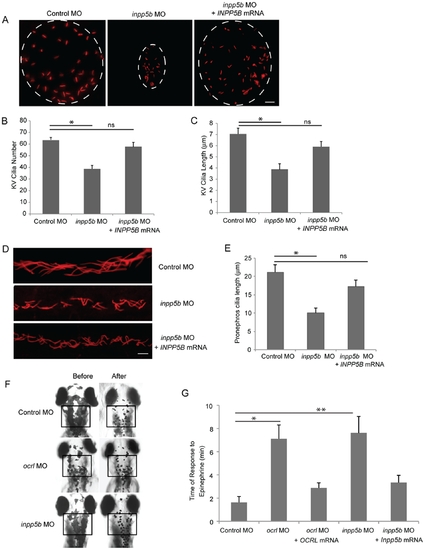
Defects of cilia formation in Inpp5b zebrafish morphants. (A) INPP5B WT mRNA rescue the loss of inpp5b. KV cilia of zebrafish embryos injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and INPP5B WT mRNA (500 ng) at 6-somite stage were immunostained with acetylated α-tubulin (red), representative images are shown (dash line indicates border of KV). Scale bar 10 micron. (B–C) Quantification of number (B) and length (C) of KV cilia in zebrafish embryos injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and INPP5B WT mRNA (500 ng). (N >20 embryos, three independent experiments, unpaired t-test, * p = 3.4E-03 in B and * p = 1.9E-23 in C). (D) INPP5B WT mRNA rescue of inpp5b pronephric cilia formation. Representative image of pronephric cilia of zebrafish embryos at 24 hpf stage, injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and INPP5B WT mRNA (500 ng), immunostaining with acetylated α-tubulin (red). Scale bar 10 micron. (E) Pronephric cilia length of control and inpp5b MO. Pronephric cilia of zebrafish embryos injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and INPP5B WT mRNA (500 ng) at 24 hpf stage were analyzed by immunostaining with acetylated alpha-tubulin and cilia length was measured. (N >200 cilia; three independent experiments, unpaired t-test, * p = 5.45E-18). (F) Ocrl and Inpp5b morphants showed slowed retrograde melanosome transport. Representative photos are shown for the melanosomes in Ocrl and Inpp5b morphants before and after treatment with epinephrine in 5 dpf embryos (box, region of pigment evaluation). (G) Quantification of the response time for epinephrine treatments in the control MO (2 ng), ocrl MO (2 ng), ocrl MO (2 ng) and OCRL WT mRNA (500 pg), inpp5b MO (2 ng), and inpp5b MO (2 ng) and Inpp5b WT mRNA (500 pg) embryos (N >30 embryos, three independent experiments, unpaired t-test, * p = 4.6E-50, ** p = 2.3E-43). PHENOTYPE:
|
|
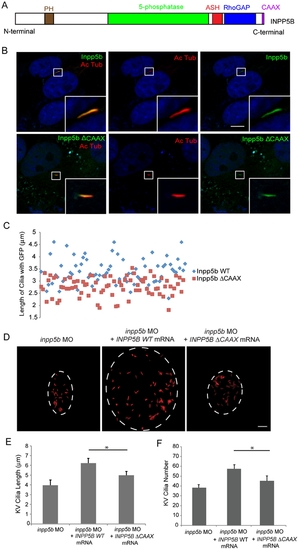
Effect of INPP5B CAAX mutant on cilia localization. (A) Domain structure of human INPP5B protein. (B) hTERT-RPE1 cells were transduced by GFP-Inpp5b or GFP-Inpp5bΔCAAX lentivirus, starved for 48 hr, and then analyzed by immunostaining with anti-acetylated alpha-tubulin antibody. Scale bar 10 micron. (C) Lengths of primary cilia in Inpp5b and Inpp5b-delta-CAAX cells. hTERT-RPE1 cells were transduced with either GFP-Inpp5b or GFP-Inpp5b-delta-CAAX lentivirus, serum starved for 48 hr, and stained with anti-acetylated alpha-tubulin antibody. Scatter plot showing distribution pattern of ciliary length (3.4±0.4 micron in INPP5B and 2.8±0.3 micron in Inpp5bΔCAAX cells, unpaired t-test, p = 1.36E-06, n >160 cilia, three independent experiments). (D) INPP5BΔCAAX mRNA failed to rescue the loss of inpp5b. KV cilia of zebrafish embryos injected with inpp5b MO (4 ng), inpp5b MO (4 ng) with INPP5B WT mRNA (500 ng) and inpp5b MO (4 ng) with INPP5B ΔCAAX mRNA (500 ng) at 6-somite stage were immunostained with acetylated α-tubulin (red), representative images are shown (dash line indicates border of KV). Scale bar 10 micron. (E–F) Quantification of length (E) and number (F) of KV cilia in zebrafish embryos injected with inpp5b MO (4 ng), inpp5b MO (4 ng) with INPP5B WT mRNA (500 ng) and inpp5b MO (4 ng) with INPP5B ΔCAAX mRNA (500 ng). (N >20 embryos, three independent experiments, unpaired t-test, * p = 3.9E-26 in E and * p = 9E-06 in F). PHENOTYPE:
|
|
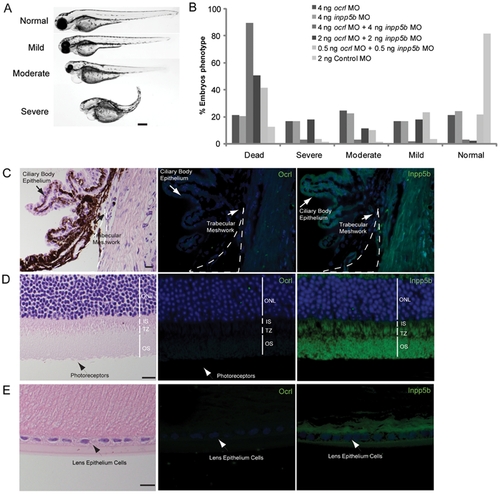
INPP5B and OCRL act synergistically in eye development. (A) Representative phenotypes of morphants co-injected with ocrl and inpp5b MO. Scale bar 250 micron. (B) Dose-dependent effect of two morpholinos in zebrafish. Control ocrl and inpp5b MO at indicated doses were injected into zebrafish embryos, and different grades of phenotypes were quantified at 48 hpf (N >250 embryos per group). (C) Transgenic mOcrl-/-:mInpp5b-/-:hINPP5B+/+ mouse eyes were sectioned and stained by H&E, anti-OCRL antibody (green) or anti-INPP5B antibody (green), and DAPI (blue). Staining of trabecular meshwork cells in vesicular pattern (arrow), dash line indicates border of cornea and iris. Scale bar 10 micron. (D) Photoreceptor cells (arrow) from the transgenic mouse eye section stained with H&E, anti-OCRL antibody (green) or anti-INPP5B antibody (green), and DAPI (blue). Scale bar 10 micron. (E) Lens epithelial cells (arrow) stained with H&E, anti-OCRL antibody (green) or anti-INPP5B antibody (green), and DAPI (blue). Scale bar 10 micron. PHENOTYPE:
|
|
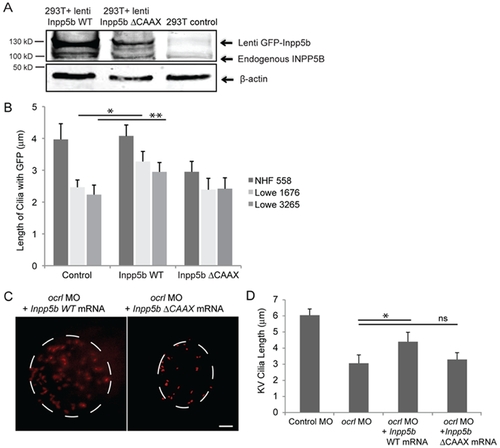
INPP5B can partly rescue the defect of OCRL in primary cilia development. (A) INPP5B protein levels in HEK293T cells. GFP-Inpp5b lentivirus was generated in HEK293T cells; both GFP-Inpp5b and endogenous INPP5B were immunoblotted in 40 microgram lysates; beta-actin levels are shown. (B) NHF 558, Lowe 1676, and Lowe 3265 fibroblasts were transduced with control, Inpp5b or Inpp5b ΔCAAX lentivirus, serum-starved for 48-hours, and immunostained with acetylated alpha -tubulin. Quantification of cilia length is shown (n >100 cilia, three independent experiments, unpaired t-test, * p = 0.004, ** p = 0.006). (C) Inpp5b mRNA partly rescued the loss of Ocrl. KV cilia of zebrafish embryos injected with ocrl MO (4 ng) with Inpp5b WT mRNA (500 ng) and ocrl MO (4 ng) with Inpp5bΔCAAX mRNA (500 ng) at 6-somite stage were immunostained with acetylated α-tubulin (red), representative images are shown (dash line indicates border of KV). Scale bar 10 micron. (D) Quantification of length of KV cilia in zebrafish embryos injected with ocrl MO (4 ng) with Inpp5b WT mRNA (500 ng) and ocrl MO (4 ng) with Inpp5bΔCAAX mRNA (500 ng). (N >20 embryos, three independent experiments, unpaired t-test, * p = 2.2E-04). PHENOTYPE:
|
|
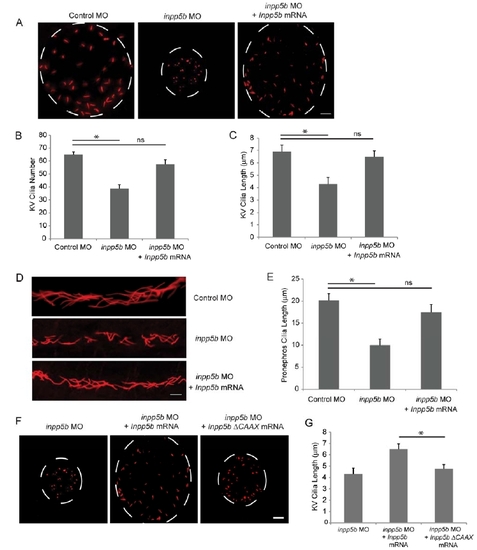
Restoration of cilia defects in Inpp5b zebrafish morphants by mouse Inpp5b mRNA. (A) Inpp5b WT mRNA rescued the loss of inpp5b. KV cilia of zebrafish embryos injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and Inpp5b WT mRNA (500 ng) at 6-somite stage were immunostained with acetylated alpha-tubulin (red), representative images are shown (dash line indicates border of KV). Scale bar 10 micron. (B-C) Quantification of number (B) and length (C) of KV cilia in zebrafish embryos injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and Inpp5b WT mRNA (500 ng). (D) Inpp5b WT mRNA rescue of inpp5b pronephric cilia formation. Representative image of pronephric cilia of zebrafish embryos at 24 hpf stage, injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and Inpp5b WT mRNA (500 ng), immunostaining with acetylated α-tubulin (red). Scale bar 10 micron. (E) Pronephric cilia length of control and inpp5b MO. Pronephric cilia of zebrafish embryos injected with control MO (4 ng), inpp5b MO (4 ng) or inpp5b MO (4 ng) and Inpp5b WT mRNA (500 ng) at 24 hpf stage were analyzed by immunostaining with acetylated alpha-tubulin and cilia length was measured. (F) Inpp5b-delta-CAAX mRNA failed to rescue the loss of inpp5b. KV cilia of zebrafish embryos injected with inpp5b MO (4 ng), inpp5b MO (4 ng) with Inpp5b WT mRNA (500 ng) and inpp5b MO (4 ng) with Inpp5b-delta-CAAX mRNA (500 ng) at 6-somite stage were immunostained with acetylated alpha-tubulin (red), representative images are shown (dash line indicates border of KV). Scale bar 10 micron. (G) Quantification of length of KV cilia in zebrafish embryos injected with inpp5b MO (4 ng), inpp5b MO (4 ng) with Inpp5b WT mRNA (500 ng) and inpp5b MO (4 ng) with Inpp5b-delta-CAAX mRNA (500 ng) (6.5+0.5 micron in morphants of inpp5b MO with Inpp5b mRNA compared to 4.7+0.4 micron in morphants of inpp5b MO with Inpp5b-delta-CAAX mRNA, unpaired t-test, * p = 4.9E-07, N >20 embryos, three independent experiments). |