- Title
-
CERKL Knockdown Causes Retinal Degeneration in Zebrafish
- Authors
- Riera, M., Burguera, D., Garcia-Fernàndez, J., and Gonzàlez-Duarte, R.
- Source
- Full text @ PLoS One
|
Human and zebrafish CERKL protein domains. (A) The reported hCERKL (NP_963842) protein domains described by either sequence homology (PH, pleckstrin homology; DAGK, diacylglycerol kinase and ATPbs, ATP binding site domains) or by functional analysis (NLS, nuclear localization signals; NES, nuclear export signals) and their conservation in zebrafish is shown in percentage of identity. (B–D) ZFCerkl-HA shares with human CERKL the dynamic subcellular localization in COS-7 transfected cells, shifting from the cytoplasm to the nucleus. Nuclei were stained with DAPI. Images correspond to individual optical sections. Photographs were at×63 magnification. (B) In most cells, ZFCerkl shows a uniform distribution in the cytosol and is absent from the nucleus. (C) Some cells per field showed localization of Cerkl in both, the cytosol and the nucleus, with clear exclusion from the nucleoli (white arrow). (D) Rarely, Cerkl contributes to cytosolic aggregates. |
|
Expression of cerkl transcripts during embryonic development and adult tissues. (A) Temporal and spatial expression of zebrafish (Dre) cerkl assessed by RT-PCR (top) and RNA-seq data retrieved from databases (bottom) at different developmental stages and adult tissues. (B and C) Expression of cerkl in developmental stages and tissues of frog (Xtr) and chicken (Gga). hpf, hours post-fertilisation; dpf, days post-fertilization; st, stage. EXPRESSION / LABELING:
|
|
Cerkl in situ hybridization on embryo and adult zebrafish. (A and B) Whole-mount RNA in situ hybridization analysis showing cerkl expression in the retina and brain of embryos at 24 and 50 hpf. R, retina; L, lens. (C and D) In situ hybridization on zebrafish retina cryosections of 72 hpf embryos and adult tissue. Cerkl expression is detected in the three nuclear layers of the embryo retina, whereas adult expression appears in the inner segment of the photoreceptors and some cells located at the basal layer of the INL. Positive control (antisense CRX), strongly labels the inner photoreceptor segment and inner nuclear layer. The negative control was performed with sense cerkl. RPE, retinal pigment epithelium; PR, photoreceptor; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. |
|
Effects of ZFcerkl silencing in eye development. (A) Two morpholinos targeting an acceptor (acMO) and donor splice site (doMO) of ZFcerkl were used. The morpholino targeting site is depicted by a discontinuous line. To assess the knockdown effect, a RT-PCR analysis of control and ZFcerkl morphants injected with 8 ng of MO was performed (primers used are depicted with arrows). Silencing with acMO was almost complete, when compared with cerkl expression in 72 hpf control MO-injected embryos (coMO), whereas in doMO animals the WT spliced isoform was decreased by 35%. β-Actin was used for normalization. (B) acMO-injected embryos displayed either small eye phenotype (named mild phenotype) or very small eye, small head, and body and curved tail (named severe phenotype). White double-head arrows denote diameter of the eyes. * denotes small and curved head in severe-phenotype morphants. (C) Phenotype frequency of morphants. The cerkl-knockdown phenotype was rescued when human CERKL mRNA was co-injected with acMO. n, number of individuals. (D) Eye size (in diameter) was measured in at least ten independent embryos from each group. Data were analyzed by t-test and are presented as mean ± SEM. ** P<0.001. Mean eye size was 253.4 µm for control, 172.7 μm for acMO and 239 μm for doMO morphants. PHENOTYPE:
|
|
Eye histology in control and ZFcerkl morpholino-injected embryos at 72 hpf. (A) Zebrafish eye sections were stained with H&E. Control morphants (coMO) showed normal retinal lamination with three cell layers (GCL, INL, and ONL). In “mild” acMO-injected embryos, lamination did not occur and the three layers were not visible. The retinal pigment epithelium (RPE) developed normally in control and ZFcerkl morphants. (B) Immunostaining with anti-rhodopsin and (C) anti-PNA identified rod and cone outer segments, respectively, in control and doMO morphants. Outer segments were absent in acMO morphants. Nuclei were stained with DAPI. Some nonspecific staining was seen in the lens when stained with PNA. Photographs were at×40 magnification. PHENOTYPE:
|
|
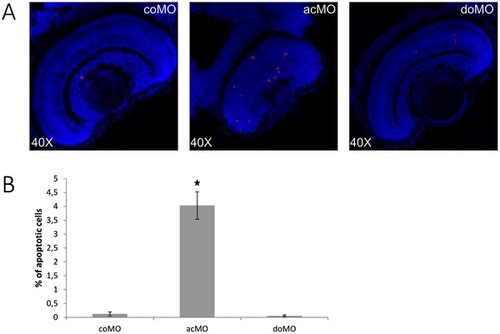
Increased cell death in ZFcerkl morpholino (acMO)-injected embryos. (A) Immunodetection of apoptosis by anti-active Caspase-3 in retina cryosections of 72 hpf control (coMO) and ZFcerkl morpholino-injected embryos (acMO and doMO). Caspase-3-positive cells (shown in red) increased in acMO morphants. Nuclei were stained with DAPI. (B) The percentage of apoptotic cells in each retina within a single cell layer was quantified in four independent embryos from each group, plotted and analysed by t-test. Data are presented as mean ± SEM. * P = 0.005. PHENOTYPE:
|
|
Expression of retina cell markers in acMO-injected embryos at early developmental stages. (A and B) At 22 and 24 hpf, the spatiotemporal pattern of pax6a (A) and otx2 (B) in acMO-injected animals was similar to that of controls: pax6a was detected in the forebrain, hindbrain, spinal cord and eye, and otx2 in the eye and midbrain. By 48 and becoming more evident at 72 hpf, acMO embryos exhibited a marked reduction in the expression of both markers. (C) The expression of the ath5 transcription factor was assessed in vivo in acMO-injected embryos of the transgenic ath5:GFP strain. At 48 hpf, the wave of ath5 expression, which prefigures the wave of retinal ganglion cell genesis, filled the central and peripheral retina of control embryos, whereas the pattern appeared delayed and disorganized in the acMO morphants, although RGC genesis was not fully abolished. * denotes the ventronasal patch of RGC genesis. EXPRESSION / LABELING:
|
|
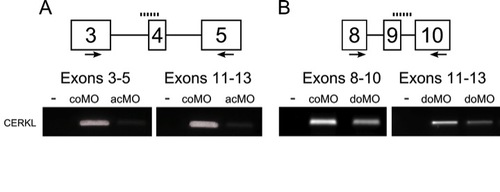
Validation of cerkl morpholinos. Transcriptional products obtained with the following sets of primers: (A) exons 3-5 and 11-13 for acMO samples, and (B) exons 8-10 and 11-13 for doMO samples. The comparable decrease in band intensity suggests transcript depletion in both cerkl morphants. |