- Title
-
A Functional Requirement for PAK1 Binding to the KH(2) Domain of the Fragile X Protein-Related FXR1
- Authors
- Say, E., Tay, H.G., Zhao, Z.S., Baskaran, Y., Li, R., Lim, L., and Manser, E.
- Source
- Full text @ Mol. Cell
|
The KH2 Domain of FXR1 Is Implicated in PAK1 Binding (A) Constructs encoding the N-terminal domain of human FMR1(1–380), FXR1(1–390), FXR2(1–380), or zebrafish Zf-FXR1(1–380) were expressed in COS-7 cells and lysates passed through GSH Sepharose preloaded with AID sequences derived from human PAK1, PAK2, or PAK3 as indicated. The presence of fragile X proteins was shown by western blotting with anti-HA. The blot shown represents a single panel. (B) A schematic of the domain structure of PAK kinases showing the disposition of Cdc42-binding CRIB domain, autoinhibitory AID, and PIX-binding region. The region of PAK1 required for binding FXR1 is shown, with those residues that are identical between the three isoforms shaded. (C) The KH(2) domain of FXR1 is implicated in PAK1 binding. HA-tagged versions of fragile X-related proteins were co-expressed with the GST-PAK1-AID as shown. The presence of the HA-tagged protein (bottom) and in the GSH Sepharose pull-down (top) indicates that KH2 FXR1(284–380) is sufficient to allow PAK1 binding. See the lane FXR2(1–296)-FXR1(284–380) designated chimaera 2. |
|
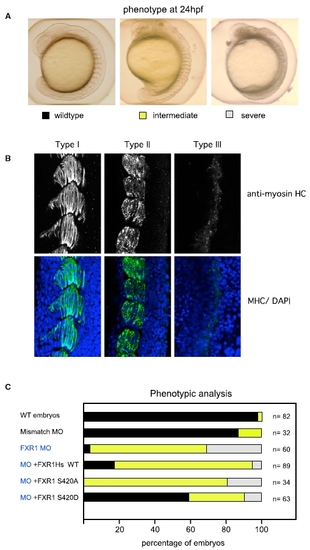
Rescue of FXR1 Depletion in Zebrafish Using Human FXR1 mRNA (A) Zebrafish embryos injected with synthetic morpholino-oligonucleotides (MO) against Zf FXR1; 2.5 ng was the lowest concentration that gave a high penetrance of phenotype (>90%). The organization of somites observed by bright field microscopy were scored as normal (black) when the chevron-shaped structures were clearly visible; intermediate (yellow) when the somites were clearly segmented but the shape abnormal; or severely disrupted (gray) when somite boundaries were poorly defined. (B) FXR1 leads to disorganization of muscle fibers. Muscle organization in the three classes of embryos illustrated in (A) was assessed by myosin heavy-chain (HC) staining and confocal imaging (5 μM section), with nuclei counterstained by DAPI. (C) Rescue of FXR1 loss by co-injection at the one-cell stage. Analysis of the phenotypes observed in embryos injected with FXR1 MO and co-injection with mRNA (100 ng) encoding wild-type FXR1, FXR1(S420A), or phospho-mimetic FXR1(S420D). The color coding for the bars indicates the embryos illustrated in (A). |
|
The PAK1 Interaction Is Required for the Physiological Function of FXR1 (A) FXR1(Q348K/E352A) cannot rescue developmental defects in muscle development in zebrafish. Assays were performed as detailed in Figure 4. Synthetic mRNA encoding FXR1(I304N) or FXR1(Q348K/E352A) could not rescue the somite defects associated with loss of FXR1. The human FMR1, although able to bind PAK1, was unable to rescue FXR1 loss, indicating different requirements in terms of muscle development. (B) Confocal images of embryos treated with FXR1 MO (1.5 ng per embryo) or in combination with the PAK1 inhibitor IPA3 (10 μM) added at 7 hpf (i.e., after gastrulation). Representative images show organization of myosin heavy chain (at 24 hpf); IPA3 treatment enhances the severity of the effects of FXR1 loss. (C) Rescue of FXR1 MO-mediated knockdown using a triple mutant (TM) PAK1 binding-deficient FXR1(Q348K/E352A/S420D) can allow a gain of function, unlike the FXR1(Q348K/E352A) = KA. The panels show typical phenotypes of three morphant and three FXR1-TM rescue embryos at 24 hpf. Note the clear somite boundaries observed in the case of rescued embryos. In these experiments, quantification of the phenotypes in embryos was carried out after embryos were selected at 6 hpf for equal injection of MO and mRNA co-injected with Texas-red dextran. The table indicates the percentage of embryos in each category scored as for (B), with n being the number of embryos scored for each injection. PHENOTYPE:
|