- Title
-
Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling
- Authors
- Jülich, D., Mould, A.P., Koper, E., and Holley, S.A.
- Source
- Full text @ Development
|
Itgα5-GFP clustering and FN matrix assembly during somitogenesis in zebrafish. (A-D) Four time points (indicated in minutes) showing Itgα5-GFP localization during somite formation. Itgα5-GFP (green) is distributed along the cortex in mesenchymal presomitic cells (A) but clusters to the basal side of columnar border cells (B-D, arrows). Nuclei are red. (E-G)Itgα5-GFP (E), FN (F) and overlay (G). Nascent borders show Itgα5-GFP clustering (arrowheads in E) but no FN immunostaining. (H,I) Three-dimensional reconstruction showing FN matrix along the surface of the paraxial mesoderm. (A-H) Dorsal views, anterior is up. (I) Rotation of H showing a transverse view of the presomitic mesoderm, dorsal is up. Embryos are at the 8- to 10-somite (A-D,H,I) or 5- to 6-somite (E-G) stage. n, notochord; s, somites. Scale bars: 30 μm. EXPRESSION / LABELING:
|
|
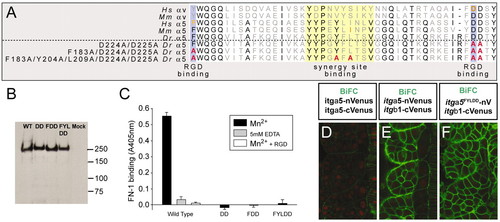
Ligand-binding-deficient variants of Itgα5. (A) An alignment of a portion of the β-propeller domain of human (Hs), mouse (Mm) and zebrafish (Dr) Integrin α5 and αV. Residues known to be necessary for RGD binding (blue) and synergy site binding (yellow) are indicated. The orange residues have been analyzed experimentally in the corresponding Integrin. The sequences of the modified zebrafish Itgα5 are shown with the altered residues in red. (B) Western blotting of wild-type (WT) and mutant (DD, FDD and FYLDD) recombinant-soluble α5β1-Fc Integrins, showing that in each case a heterodimer is formed of ∼240 kDa. This band is not observed in proteins purified from the supernatants of mock-transfected cells. (C) Ligand-binding assay. Binding of a recombinant fragment of zebrafish Fn1 to wild-type or mutant (DD, FDD and FYLDD) α5β1-1-Fc was measured in a solid-phase assay in the presence of 1 mM Mn2+ to activate the Integrin (black bars). To demonstrate specificity of Fn1 binding to the wild-type Integrin, the interaction was also measured in the presence of cyclic RGD peptide (white bar) or 5 mM EDTA (gray bar). (D-F) BiFC demonstrates that the mutant Itgα5FYLDD forms a heterodimer with Itgβ1 on the cell cortex in vivo. Negative (D) and positive (E) controls are also shown. Anterior is up and medial is to the right. |
|
Initial clustering of Itgα5-GFP is independent of FN. (A)In vitro transcribed mRNAs encoding Itgα5-GFP variants were injected at 250 ng/μl into zebrafish embryos derived from Itgα5-/+ parents. Injected embryos were assayed morphologically for somite defects, for antimorphic activity and for Itgα5-GFP clustering. (B-G) Lateral views of the trunk somites in 14- to 18-somite stage embryos. Anterior is left. (B) A wild-type (wt) embryo. (C) An itga5-/-embryo. Somitogenesis in itga5-/- is not rescued by injection of mRNA encoding Itgα5DD-GFP (D), Itgα5FDD-GFP (E) or Itgα5FYLDD-GFP (F). The itga5-/- embryo in C is an uninjected sibling of the embryo in F. Note that the morphological somite defects are enhanced by Itgα5FYLDD expression. (G) Loss of maternal itga5 enhances the zygotic itga5-/- phenotype; however, posterior trunk and tail somites form in these embryos. (H-O) Embryos at the 6- to 8-somite stage. Itgα5DD-GFP does not rescue segmental FN assembly in itga5-/- embryos (H) but clusters along nascent borders (I). Similarly, Itgα5FDD-GFP does not rescue segmental FN assembly in itga5-/- embryos (J) but clusters along nascent borders (K). (L) MZ itga5-/- embryos lack segmental FN. FN localization in itga5-/- (M) and an itga5-/- embryo injected with Itgα5FYLDD (N). (O) Itgα5FYLDD-GFP localization in the embryo shown in N. Note that the FN-matrix defects are enhanced by injection of Itgα5FYLDD, with matrix only forming along the surface of the paraxial mesoderm (asterisks). Nonetheless, Itgα5FYLDD-GFP clustering is observed (arrowhead). (P,Q) Two time points (indicated in minutes) of Itgα5FYLDD-GFP localization in an 8- to 10-somite stage embryo lacking maternal and zygotic itga5 (MZ itga5-/-). A functional ligand-binding domain is not necessary for initial clustering (arrows) but is required to maintain clustering. For reference, the same two cells are outlined in yellow and white. (H-Q) Anterior is up. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Itgα5 non-cell-autonomously inhibits clustering and FN matrix assembly. (A) Genetic mosaics were made by transplanting labeled cells (red) from the blastula of a donor zebrafish embryo into the late blastula of an unlabeled host embryo. The chimeras were later analyzed for morphological border formation, greater than three cell diameters in length, around clones within the paraxial mesoderm. (B) Summary of the genetic mosaic experiments. Donor and host genotypes and treatments are indicated. The percentage of clones with borders, the total number of embryos examined and the number of experiments are displayed. (C,D) FN matrix (C) is eliminated by injection of fn1/fn1b morpholinos (D). FN matrix is blue and nuclei are red. (E,F) DIC image (E) and composite of a clone (F, red) with a border separating it from the host cells. (G) FN matrix forms along a clone lacking fn but expressing itga5 within a host lacking itga5 but expressing fn. Asterisks indicate FN matrix along the medial and lateral surfaces of the paraxial mesoderm. (H) FN matrix forms along a clone lacking itga5 within a host expressing itga5. (I) In mosaics of the same genotype as in H, Itgα5-GFP clusters along the clone. (J) The boxed region in I shown with FN matrix (blue) colocalizing with the clustered Itgα5-GFP. Note the columnar morphology of the host border cells. (K) Actin belts are seen along the clone borders. (C-K) Anterior is up. |
|
Eph/Ephrin signaling can induce Itgα5 clustering and FN matrix assembly. (A-D) Phosphorylated Epha4 (Epha4-P-Tyr) localizes to somite boundaries and the notochord (n) in wild-type zebrafish embryos (A) but is substantially reduced in epha4 morpholino-injected embryos (B). Ephrin B2a (green) is expressed in a graded fashion in the posterior somites (arrows) of wild-type embryos (C) but is absent in ephrin B2a morpholino-injected embryos (D). Nuclei are red. (E) Epha4-P-Tyr localizes to transient somite boundaries (arrows) in MZ itga5-/- embryos. (F-H) Epha4-P-Tyr blue) colocalizes with ligand-independent Itgα5FYLDDD-GFP clustering (arrows) in MZ itga5-/- embryos. (I,J) Morpholino knockdown of ephrin B2a abolishes ligand-independent Itgα5FYLDD-GFP clustering. (K-M) epha4-expressing clones (red) in a host with no epha4 but with broadly expressed ephrin B2a. Eph/Ephrin signaling can induce a border (K) and Itgα5-GFP clustering (L,M) around the donor clone. Note that Itgα5-GFP in the host cells is largely localized to the border with the clone (arrows), not along the lateral cell cortices. (N) A rosette with polarized host cells surrounding an epha4-expressing clone. Actin fibrils concentrate along the `basal′ surface of the host cells, similar to polarization along somite boundaries. (O,P) FN matrix is assembled around the epha4-expressing clone. (Q-S)A dnepha4-expressing clone (red) induces Itgα5-GFP clustering and FN matrix assembly. C,D,H,M,N,O,S are overlays. EXPRESSION / LABELING:
|
|
Impairment of itga5 function leads to a phenotype in the zebrafish head. Lateral views of 9-somite stage zebrafish embryos. DIC (A-C) and widefield (D-F) images of cell nuclei labeled with propidium iodide. (A,D) Wild type. (B,E) In itga5-/- embryos, there is a deficit of cells posterior to the eye (asterisks). This phenotype is evident in mutant embryos with virtually unrecognizable somite phenotypes (presumably due to maternal rescue and/or genetic background). Thus, head development is more sensitive than somitogenesis to the loss of itga5. (C,F) This head phenotype is observed in wild-type embryos that express itga5FYLDD, demonstrating the antimorphic activity of this integrin variant. PHENOTYPE:
|