- Title
-
Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation
- Authors
- Feng, X., Adiarte, E.G., and Devoto, S.H.
- Source
- Full text @ Dev. Biol.
|
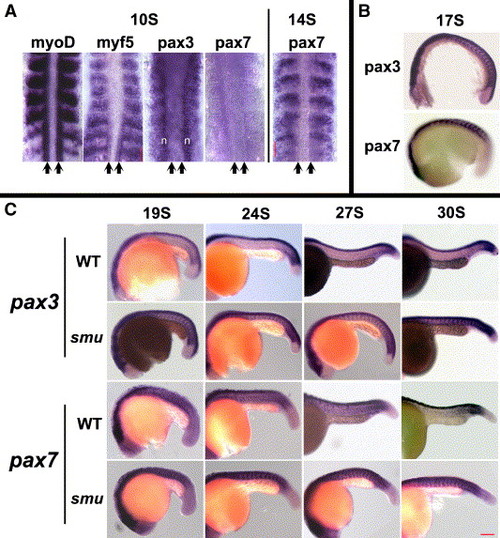
Hedgehog signaling down-regulates pax3/7 expression after the 19-somite stage. A clutch of smu+/- embryos was hybridized with pax3 or pax7 probes during early embryonic stages. (A) Dorsal view of embryos at 10S and 14S stages. At the 10S stage, myoD and myf5 are expressed in adaxial cells (arrows) and the posterior part of newly formed somites. Neither pax3 nor pax7 is expressed in adaxial cells (arrows). pax3 is expressed initially throughout the non-adaxial portion of the somite; pax3 is also expressed in the neural folds (n). pax7 is not expressed in the somites. At the 14S stage, pax7 is expressed in the anterior part of somites, at this stage the somitic expression of pax3 is very similar but has much higher expression in neural tissue. (B) Lateral view of embryos at 17S stage, the expression of pax3 and pax7 continues to be strongest in the anterior part of somites, and is indistinguishable between wild-type and smu mutant embryos. (C) After the 19-somite stage, pax3 expression is down-regulated in wild-type embryos, while the segmented expression of pax3 is maintained in embryos mutant for smu(smo), at least until the end of segmentation stage (30S). pax7 expression is also down-regulated during this period in wild-type embryos, while the segmented expression of pax7 is maintained at high levels in embryos mutant for smu(smo). Scale bar = 100 μm. EXPRESSION / LABELING:
|
|
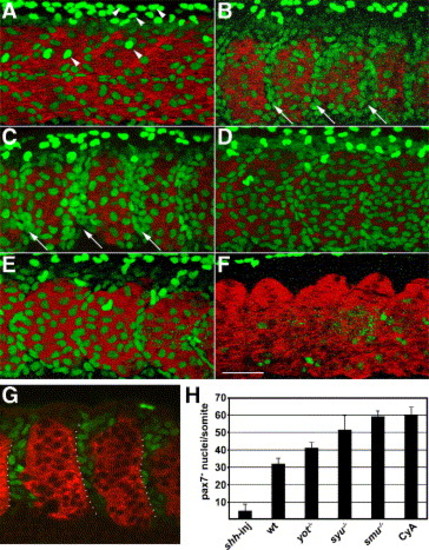
Hedgehog signaling negatively regulates the number of Pax7+ myogenic precursors. Embryos were labeled at the prim5 (24 h) stage for Pax7 (green) and myosin (MF20, red). (A) In wild-type embryos, the surface of each somite has two types of Pax7+ myogenic precursors, moderately labeled cells that are part of the somite, and more brightly labeled, dorso-ventrally elongated neural crest cell nuclei. (B, C) smu(smo)-/- and CyA-treated wild-type embryos have more Pax7+ myogenic precursors than wild-type control embryos, particularly in the anterior part of each somite (arrows). (D, E) In syu(shh)-/- and yot(gli2)-/- embryos, the number of Pax7+ nuclei is intermediate between untreated wild-type and smu(smo)-/- or CyA-treated wild-type embryos. (F) shh mRNA-injected wild-type embryos have virtually no Pax7+ nuclei. (G) A single optical slice of a CyA-treated wild-type embryo through the middle part of somites, demonstrating the gaps between myotomes that are filled with Pax7+ cells. Many of the Pax7+ cells are within the anterior of each somite (dotted outline). (H) Quantitation of the number of Pax7+ myogenic precursors per somite in each genotype or treatment (mean of 5 embryos per genotype or treatment, somites 16–18 were counted; error bar = standard deviation). Panels A–F are projections of Z-stack confocal sections (see Materials and methods), panel G is a thin optical slice, all panels are anterior to the left, dorsal up; somite 17 is shown in each panel. Scale bar = 50 μm. |
|
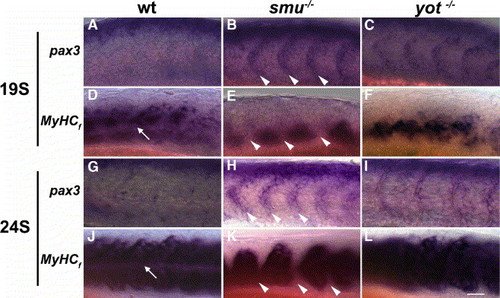
pax3 and fast MyHC gene expression show reciprocal regulation. Wild-type (A, D, G, J), smu(smo)-/- (B, E, H, K), or yot(gli2)-/- (C, F, I, L) embryos were analyzed for pax3 (A, B, C, G, H, I) and fast Myosin Heavy Chain (MyHCf: D, E, F, J, K, L), expression. By the 19-somite stage (A–F), when pax3 expression in somite anterior cells is down-regulated in wild-type embryos (A), expression of MyHCf begins to span the full anterior–posterior extent of the somite (D). In contrast, segmented expression of pax3 is maintained in anterior cells within somites of smu(smo) mutants (B), while expression of MyHCf is only found in the posterior cells of each somite (E). Arrowheads in panels B and E indicate the anterior cells of the somite, which in smu(smo) mutants express pax3 but not MyHCf. In yot(gli2) embryos (C, F), the segmented expression of pax3 is also down-regulated (C), and MyHCf is expressed in the posterior part of the somite. By the 24-somite stage (G–L), expression of MyHCf has increased in wild-type, smu(smo), and yot(gli2) mutants (J–L). However, in smu(smo) mutant embryos cells in the anterior of each somite still do not express MyHCf(K), and the segmental pax3 expression is still maintained (H). In yot(gli2) mutant embryos, anterior cells of each somite down-regulate pax3 and up-regulate MyHCf similar to wild type (I). The arrow indicates the horizontal myoseptum where muscle pioneers, not expressingMyHCf, are present in wild type. All panels are anterior to the left, dorsal up; all images are of somites 7–11. Scale bar = 100 μm. EXPRESSION / LABELING:
|
|
Wild-type slow muscle fibers do not rescue the number of Pax7+ myogenic precursors in smu(smo)-/-. A smu(smo)-/- host embryo with transplanted wild-type donor cells (blue) was labeled with antibodies for Prox1 (red) and Pax7 (green). (A) A lateral view of 6 mid-trunk somites, some of which contain donor-derived (blue), Prox1-positive (red) embryonic slow muscle fibers. (B) The same embryo labeled for Pax7, to show myogenic precursor nuclei (green), arrowheads point to neural crest cells. (C) The merged image of panels A and B, the numbers on the bottom indicate the number of Pax7+ myogenic precursor nuclei within the corresponding somite, compare to Fig. 2G. Anterior to the left, dorsal up; scale bar = 50 μm. |
|
Hh regulation of Pax7+ myogenic precursors occurs later than Hh induction of slow muscle fibers. Wild-type embryos were treated with cyclopamine from the 8-somite stage to the 30-somite (24 h) stage; they were then labeled for differentiated muscle fibers, slow muscle nuclei, and myogenic precursor nuclei. The numbers at the top of panel A indicate somite number. White lines frame the borders of somites: these were drawn solely with the MF20 labeling visible. (A) Merged image showing myosin (MF20, blue), slow muscle nuclei (Prox1, red), and myogenic precursor nuclei (Pax7, green). (B) A single color panel of A, showing Prox1-positive slow muscle nuclei in each somite, the number per somite is shown. All somites have an approximately wild-type number of Prox1-positive nuclei. Somites posterior to somite 18 had a loss of slow muscle fiber nuclei (data not shown, Hirsinger et al., 2004). (C) Single color panel of A, showing the Pax7-positive myogenic precursor nuclei in each somite. The number of Pax7+ myogenic precursors in somites 5 to 11 is similar to wild type, while posterior to somite 11, the number of Pax7+ myogenic precursors in each somite is increased to the level of smu-/-; compare to Fig. 2H. Anterior to the left, dorsal up; scale bar = 50 μm. EXPRESSION / LABELING:
|
|
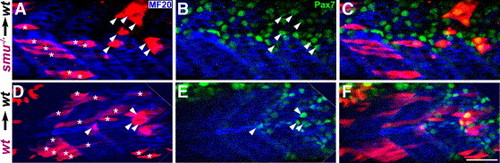
Analysis of the cell autonomy of Hh signaling requirements for Pax7+ myogenic precursor regulation. Genetic mosaics were created by transplanting smu(smo)-/- cells (A–C) or wild-type cells (D–E) into wild-type embryos, embryos were labeled for myosin (MF20, blue) and Pax7 (green). Donor-derived somite cells (red) that are elongated and presumed to be muscle are labeled with asterisks; donor-derived somite cells that are Pax7-positive are indicated with arrowheads. Hh-unresponsive cells were more likely than wild-type cells to maintain Pax7+ expression (Table I). (A) Transplanted smu(smo)-/- somitic cells (red) formed differentiated muscle fibers (arrows) as well patches of cells on the external surface of differentiated muscle fibers (arrowheads) (B) Pax7+ myogenic precursor nuclei (green) in the same optical section as panel A. (C) The merged image of panels A and B, showing that patches overlap with several Pax7+ nuclei. (D) Transplanted wild-type somitic cells (red) which have differentiated into muscle fibers (arrows) and patches of cells on the external surface of differentiated muscle fibers. (E) Pax7+ myogenic precursor nuclei (green) in the same optical section as in panel D. (F) The merged image of panels D and E, showing that the patch of transplanted cells are Pax7+ myogenic precursors. All images are single optical sections at the same magnification; anterior to the left, dorsal up; scale bar = 50 μm. |
Reprinted from Developmental Biology, 300(2), Feng, X., Adiarte, E.G., and Devoto, S.H., Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation, 736-746, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.