- Title
-
NXT2 is required for embryonic heart development in zebrafish
- Authors
- Huang, H., Zhang, B., Hartenstein, P.A., Chen, J.N., and Lin, S.
- Source
- Full text @ BMC Dev. Biol.
|
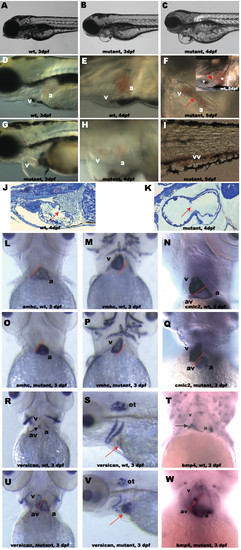
Characterization of NXT2 mutant phenotypes. At 3 dpf, NXT2 mutants show pericardial edema (compare B and G to wild-type embryos A and D). By 4 dpf, both atrium and ventricle of NXT2 mutants show significant dilation (compare H to wild-type embryo E) with pericardial sac enlargement (C and H). At 5 dpf, NXT2 mutant heart appears to be lacking atrioventricular boundary (F, arrow) and shows blood accumulation in both cardiac chambers in enlarged heart sac (F) and /or peripheral circulation (I, tail vessel) whereas the wild type heart with a normal atrioventricular boundary (arrow) resides in a narrowed cardiac sac (inset in F). Sections of wild-type (J) and NXT2 mutant (K) hearts at 4 dpf show that ventricular wall of NXT2 mutant embryos is thinner and a space separating myocardium and endocardium in atrium is visible. In addition, we often observed mutant embryos lacking valve structure (arrows). RNA whole mount in situ hybridization analyses of myocardial differentiation in wild-type (L, M, N, R, T) and NXT2 mutant (O, P, Q, U, W) at 3 dpf. All ventral view, anterior to the top except S and V, which are lateral view, anterior to the left. Solid red line marks the site of the boundary between the ventricle (v) and the atrium (a). NXT2 mutants show normal expression of cardiac chamber-specific markers amhc (L and O), vmhc (M and P), cmlc2 (N and Q). At 3 dpf, versican (R and U) and bmp4 (T and W) are restricted to the atrioventricular boundary in wild-type embryos (black arrows), but, are diffusely expressed in both cardiac chambers in NXT2 mutants. Versican expression pattern in otoliths is not changed in mutant embryos (S and V, red arrows: heart). vv: veinous vessels; a: atrium; v: ventricle; av: atrioventricular boundary; ot: otoliths. wt: wild type; mutant: zNXT2 homozygous mutant embryos; dpf: days post fertilization. EXPRESSION / LABELING:
|
|
Identification of NXT2 as a candidate gene responsible for the heart defect. Southern blot analysis shows that a 4.3 kb (digested by Hpa I; A, lane 2) and a 3.8 kb (digested by Dra I; A, lane 3) are detectable using a specific viral DNA probe (B). Lanes 1 and 4 (A) are positive and negative control, respectively. DNA fragments corresponding to 3.8 kb / Dra I and 4.3 kb / Hpa I restriction were gel-purified and used for inverse PCR to isolate junction genomic sequences with primers described in (B) and Methods. This resulted in identification of NXT2 gene, which consists of five exons (C) and encodes 143 putative amino acids (D). The provirus is inserted within the first intron (red arrow) upstream of the putative initiation codon ATG in exon 2 (C). Alignments of protein sequences from various organisms revealed that zebrafish NXT2 shares 73.9%, 72.5%, 71.6%, 70.2%, 70.9%, 68.8%, 43.6% and 34.6% amino acid homology with that of rat NXT2, human NXT2, human NXT1, rat NXT1, mouse NXT1, Xenopus NXT1, Drosophila NXT1 and C. elegans NXT1, respectively (D). Identical residues are shown in red boxes. Zebrafish NXT2 is ubiquitously expressed at 2 dpf as shown by RNA whole mount in situ hybridization (E, arrow points to heart). E: lateral view, anterior to the left. zNXT2: zebrafish NXT2; hNXT1: human NXT1; hNXT2: human NXT2; mNXT1: mouse NXT1; rNXT1: rat NXT1; rNXT2: rat NXT2; cNXT1: C. elegans NXT1; dNXT1: Drosophila NXT1 and xNXT1: Xenopus NXT1. EXPRESSION / LABELING:
|
|
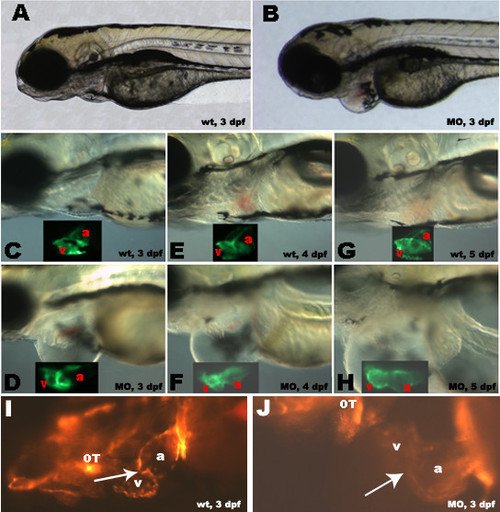
Morpholino antisense knockdown of zNXT2 in transgenic zebrafish embryos expressing cmlc2-GFP and flk-RFP. zNXT2 knockdown causes pericardial edema (compare A, C, E, G with B, D, F, H), abnormal relative positions of two chambers and chamber dilation (compare insets in C-H showing CMLC2 promoter driven GFP expression patterns in myocardium of atriums and ventricles). Observation of endothelial cells at the atrioventricular boundary in living flk-1- RFP transgenic embryos allowing visualization of cardiac valve formation (I and J). Clustering of endothelial cells was clearly visible in the cardiac valve region in wild-type embryos (I, arrow) whereas injection of zNXT2 morpholino caused a failure of clustering of endocardial cells at the atrioventricular boundary (J). All embryos are lateral view, anterior to the left. a: atrium; v: ventricle; OT: outflow tract; A, C, E, G: uninjected wild-type embryos; B, D, F, H: morpholino-injected embryos. wt: wild type; dpf: days post fertilization; MO: zNXT2 morpholino. |