- Title
-
A noncanonical sequence phosphorylated by casein kinase 1 in {beta}-catenin may play a role in casein kinase 1 targeting of important signaling proteins
- Authors
- Marin, O., Bustos, V.H., Cesaro, L., Meggio, F., Pagano, M.A., Antonelli, M., Allende, C.C., Pinna, L.A., and Allende, J.E.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
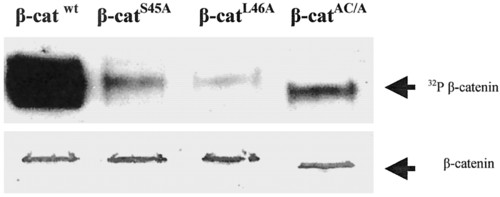
The phosphorylation of full-length β-catenin WT and mutants by CK1α. The recombinant, bacterially expressed, (His)6-tagged α-catenin WT and mutants from zebrafish were phosphorylated in vitro with the homologous CK1αL enzyme as described in Materials and Methods. The mutants included Ser-45 replaced by alanine (S45A), Leu-46 replaced by alanine (L46A), and all components of the acidic cluster (E53, D54, E55, D56, and D58) changed to alanine (AC/A). (Upper) Bands are autoradiography of the 32P incorporated into β-catenin after SDS/PAGE fractionation. (Lower) Band represents the Western blot of the (His)6 β-catenin present that was developed with monoclonal anti-(His)6 antibody. |