Lab
Rachel K. Miller Lab
|
|
Statement of Research Interest
Numerous organ systems require development of tubular structures, including the gastrointestinal, urogenital and respiratory systems. Tubes enable transport, absorption and secretion of fluids and gases. Development of organs with precise structures requires coordination of cell movements. We are focusing on in mechanisms by which cells are shaped and morphogenesis is coordinated to produce defined tissue forms.
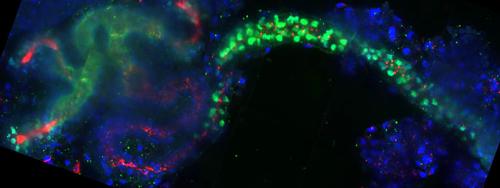
We utilize frog (Xenopus) embryonic kidneys and mammalian cultured cells as models. The tubules and duct of the nephron form the simplest unit of filtration in the kidney. While mammalian kidneys possess one million nephrons, each frog embryonic kidney has only one nephron, providing a simplified system to study organogenesis. Knockdown strategies and confocal imaging are used to assess tubulogenesis.
Altered Wnt signaling results in kidney pathologies including cystic kidney diseases and cancer. Our studies focus on how Wnt signaling modulates cell shape and organization to facilitate the formation of nephric tubules. Cells perceive their orientation within a tissue through a specific Wnt signaling pathway called planar cell polarity (PCP). Our studies focus on how PCP components with cytoskeletal roles regulate tubulogenesis.
Cystic kidneys are the most common feature of a group of genetic diseases known as ciliopathies. More than 20 of these disorders have been identified, and they are characterized by developmental defects causing blindness, deafness, chronic respiratory infections, kidney disease, heart disease, infertility, obesity, diabetes, polydactyly and situs inversus among others. Ciliopathies result from defects in cilia, including the primary cilia projecting into nephron lumens. Primary cilia act as cellular antennae, orchestrating cellular signaling and orienting cells through PCP. We are interested in the role of PCP and primary cilia in shaping nephric tubules.
Current interests in the lab include:
1) Determining how PCP components affect tubule and cilia formation
2) Discovering novel components affecting nephron development
3) Visualizing in vivo tube formation using advanced live imaging techniques
4) Generating transgenic animals to visualize nephrogenesis in vivo
We utilize frog (Xenopus) embryonic kidneys and mammalian cultured cells as models. The tubules and duct of the nephron form the simplest unit of filtration in the kidney. While mammalian kidneys possess one million nephrons, each frog embryonic kidney has only one nephron, providing a simplified system to study organogenesis. Knockdown strategies and confocal imaging are used to assess tubulogenesis.
Altered Wnt signaling results in kidney pathologies including cystic kidney diseases and cancer. Our studies focus on how Wnt signaling modulates cell shape and organization to facilitate the formation of nephric tubules. Cells perceive their orientation within a tissue through a specific Wnt signaling pathway called planar cell polarity (PCP). Our studies focus on how PCP components with cytoskeletal roles regulate tubulogenesis.
Cystic kidneys are the most common feature of a group of genetic diseases known as ciliopathies. More than 20 of these disorders have been identified, and they are characterized by developmental defects causing blindness, deafness, chronic respiratory infections, kidney disease, heart disease, infertility, obesity, diabetes, polydactyly and situs inversus among others. Ciliopathies result from defects in cilia, including the primary cilia projecting into nephron lumens. Primary cilia act as cellular antennae, orchestrating cellular signaling and orienting cells through PCP. We are interested in the role of PCP and primary cilia in shaping nephric tubules.
Current interests in the lab include:
1) Determining how PCP components affect tubule and cilia formation
2) Discovering novel components affecting nephron development
3) Visualizing in vivo tube formation using advanced live imaging techniques
4) Generating transgenic animals to visualize nephrogenesis in vivo
Lab Members
