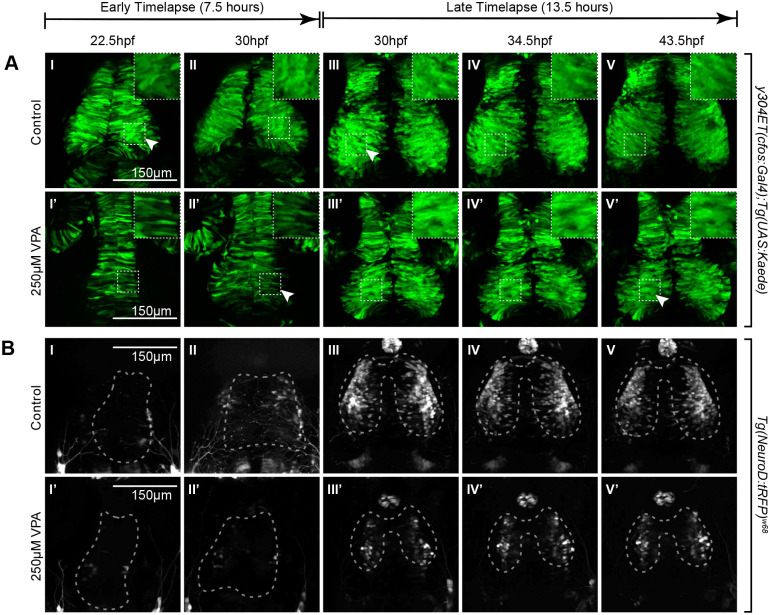
Fig. 3.
Timelapse imaging between 22.5-30 hpf and between 30-43.5 hpf shows a delay in neuronal differentiation and specification in VPA-treated embryos. (A I-II, I′-II′) Timelapse imaging of VPA-treated and control y304Et(cfos:Gal4); Tg(UAS:Kaede) embryos at 22.5hpf-30 hpf show that neuroepithelium proliferation (columnar cells) and early neuron generation/differentiation (shorter, rounder cells) is lagging in treated embryos (I′-II′) and is more comparable (arrow, II′) to controls at earlier time points (arrow, I). Insets show a magnified view of the ROIs in dashed lines. (B I-II, I′-II′) At 22.5-30 hpf, no appreciable neuronal specification is seen in either control (I-II) or treated embryos (I′-II′) as determined by lack of fluorescence in the Tg(NeuroD:tRFP)w68 line (dashed outlines indicate OT location). (A III-V, III′-V′) At 30-43.5 hpf, neuroepithelium proliferation and neuron generation/differentiation continues to lag in treated embryos (arrow, V′) when compared to controls (arrow, III). During this time, more neurons become specified in controls (B III-V) when compared to VPA-treated embryos (B III′-V′). Fluorescence in controls condenses into the shape of the OT as the timelapse progresses (B III-V). No increase in fluorescence is apparent for the treated embryos through the timelapse duration. (B III′-V′). For each timelapse, the experiment was repeated twice, and three embryos were timelapse-imaged every time. (A II-III, II′-III′; B II-III, II′-III′) Discrepancies in developmental stages between the movies of the two time periods of the same transgenic line result from delays imposed from time away from a controlled incubation environment as the timelapse progressed.