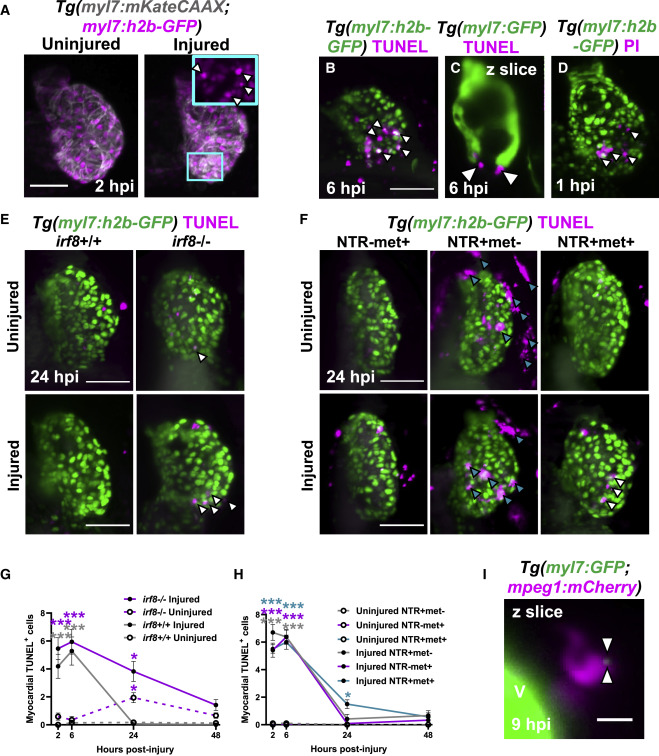
Fig. 2
Figure 2. Macrophages are required for the timely removal of apoptotic cardiomyocytes (A–C) (A) Images of uninjured and injured Tg(myl7:h2b-GFP;myl7:mKateCAAX) ventricles. Cyan outlined zoom panel highlights condensed nuclei (white arrowheads). Images of TUNEL stained hearts 6 hpi in (B) Tg(myl7:h2b-GFP) and (C) Tg(myl7:GFP) larvae. White arrowheads, apoptotic cardiomyocytes/myocardium.propidium iodide (PI)-stained
(E) Images of uninjured and injured irf8+/+ and irf8−/− Tg(myl7:h2b-GFP) ventricles stained by TUNEL at 24 hpi. White arrowheads, TUNEL+ cells.
(F) Images of uninjured and injured Tg(myl7:h2b-GFP;csfr1a:NfsB-mCherry) ventricles stained by TUNEL per macrophage ablation model injury group at 24 hpi. Cyan arrowheads, macrophages; white arrowheads, TUNEL+ cells.
(G) TUNEL+ myocardial cells in uninjured and injured, irf8+/+ and irf8−/− Tg(myl7:h2b-GFP) ventricles, n = 15–29.
(H) TUNEL+ myocardial cells in uninjured and injured Tg(myl7:h2b-GFP;csfr1a:NfsB-mCherry) ventricles per macrophage ablation group, n = 10–12.
(I) z slice of LSFM-acquired z stack, at 9 hpi, showing internalized myocardial debris (white arrowheads) in a macrophage in a Tg(myl7:GFP;mpeg1:mCherry) larva, V, ventricle-surface. Scale bars, 50 μm for (A)–(F) and 10 μm for (I). All representative images are 3D LSFM shown as maximum intensity projections unless otherwise stated. ∗ p ≤ 0.05, ∗∗ p ≤ 0.01, ∗∗∗ p ≤ 0.001. Two-way ANOVA followed by Holm-Sidak’s post-hoc tests. Data are represented as mean ± SEM.
Reprinted from Developmental Cell, 57(12), Bruton, F.A., Kaveh, A., Ross-Stewart, K.M., Matrone, G., Oremek, M.E.M., Solomonidis, E.G., Tucker, C.S., Mullins, J.J., Lucas, C.D., Brittan, M., Taylor, J.M., Rossi, A.G., Denvir, M.A., Macrophages trigger cardiomyocyte proliferation by increasing epicardial vegfaa expression during larval zebrafish heart regeneration, 1512-1528.e5, Copyright (2022) with permission from Elsevier. Full text @ Dev. Cell