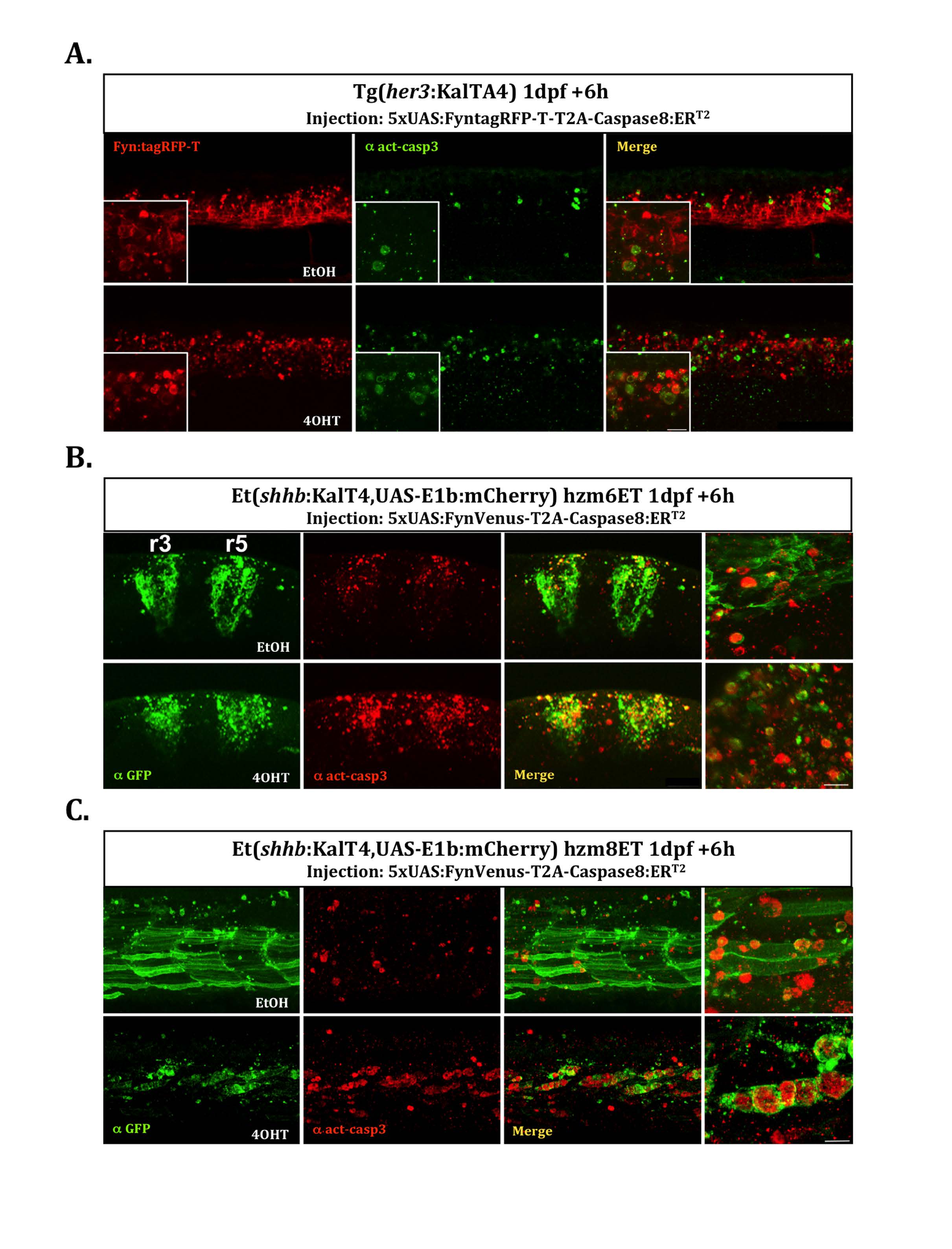
Fig. S4
Cell type specificity of apoptosis induction. Induction of apoptosis in different cells types was investigated by injection of the plasmid 5xUAS:FyntagRFP-T-T2A-Caspase8ERT2 (A) or 5xUAS:FynVenus-T2A-Caspase8ERT2 (B and C) into embryos of the different KalTA4-expressing driver strains hzm9Tg, hzm6Et and hzm8Et respectively at the one cell stage. At 24hpf injected embryos were treated for 6 hours with either EtOH (upper row) as control or 4OHT (lower row) to induce apoptosis. Subsequently, the embryos were fixed in 4%PFA/PBS and analyzed by immunohistochemistry against activated Caspase3 as indicator of apoptosis. Due to a reduced stability of FynVenus upon fixation, FynVenus expression was enhanced by immunohistchemistry using an anti-GFP antibody. Sacle bar: 10μm (insets and highly magnified images). (A) Tg(her3:KalTA4)hzm9 embryos injected with 5xUAS:FyntagRFP-T-T2A-Caspase8ERT2 reveal FyntagRFP-T-expressing red fluorescent cells in the ventral spinal cord with elevated levels of apoptosis marked by green fluorescent detection of activated Caspase3 compared to EtOH-controls. (B) hzm6Et embryos driving KalTA4-expression under control of the krox20 enhancer throughout rhombomere 3 and 5 (Distel et al., 2009) injected with 5xUAS:FynVenus-T2A-Caspase8ERT2 reveal FynVenus-expressing green fluorescent cells in the hindbrain demarcating rhombomere 3 and 5 with elevated levels of apoptosis marked by red fluorescent detection of activated Caspase3 compared to EtOH-controls. (C) hzm8Et embryos driving KalTA4-expression under control of an unkown skeletal muscle enhancer (Distel et al., 2009) injected with 5xUAS:FynVenus-T2A-Caspase8ERT2 reveal FynVenus-expressing green fluorescent cells in muscles along the trunk with elevated levels of apoptosis marked by red fluorescent detection of activated Caspase3 compared to EtOH-controls.