Fig. S9
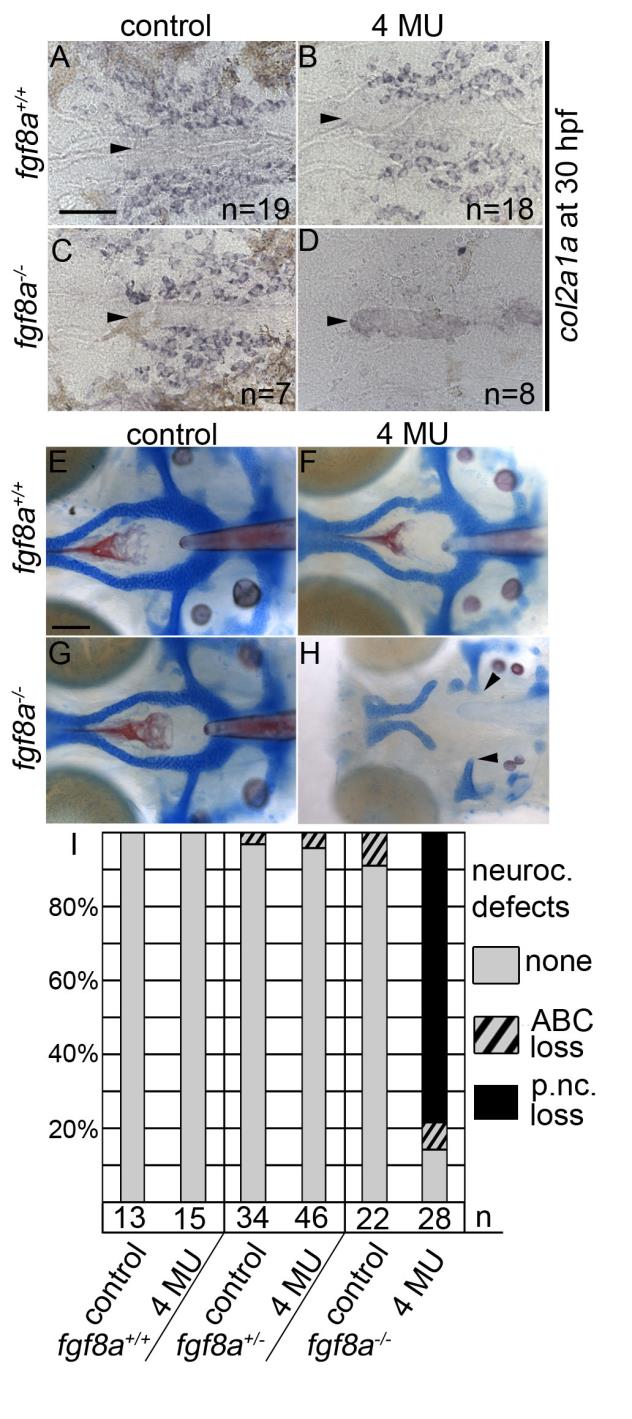
Inhibition of hyaluronic acid in fgf8a mutants causes postchordal neurocranial defects. (A-D) Images of 30 hpf embryos stained with col2a1a riboprobe. Dorsal view, with anterior to the left. (E-H) Wholemount zebrafish neurocrania at 5 days post fertilization, with the viscerocrania removed. Anterior is to the left. (A) Control-, (B) 4-MU-treated wildtype and (C) control-treated fgf8a siblings display normal col2a1a staining in the pre-postchordal neurocranium, however, (D) 4-MU-treated fgf8a mutants show a loss of col2a1a expression at the end of the treatment window (10-30 hpf). (E) Control- and (F) 4-MU-treated wildtype from 10 to 30 hours post fertilization, and (G) untreated fgf8a mutants have normal posterior neurocrania. However, (H) the mesoderm-derived regions of the neurocrania are lost in 4-MU treated fgf8a mutants (arrowheads). (I) Quantification of postchordal neurocranial defects including none (see E), ABC loss, or variable postchordal neurocranial loss (p. nc. loss) in control and 4-MU-treated wildtype (fgf8a+/+), heterozygous (fgf8a+/-) and mutant (fgf8a-/-) embryos. abc- anterior basicapsular commissure, n- notochord, oc- occipital arch, pc- parachordals, pbc- posterior basicapsular commissure. Scale bar= 50 µm in A, 20 µm in E.
Reprinted from Developmental Biology, 415(2), McCarthy, N., Sidik, A., Bertrand, J.Y., Eberhart, J.K., An Fgf-Shh signaling hierarchy regulates early specification of the zebrafish skull, 261-77, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.