Fig. 3
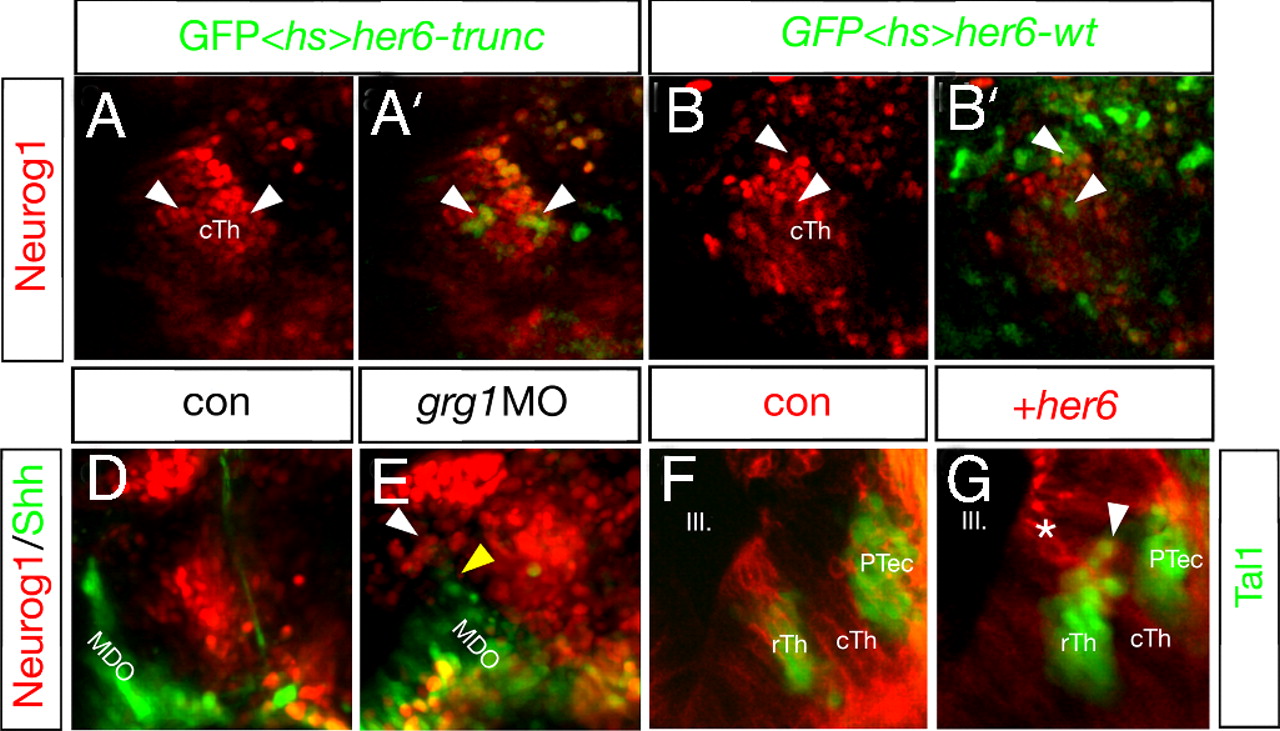
Maintenance of Her6 is required of fate of the rTh. Mis-expression of her6 represses neurog1 expression and activates rostral thalamic fate. Activation of a heat-shock inducible construct driving her6 and the lineage tracer GFP leads to the cell-autonomous down-regulation of neurog1:RFP at 33 hpf (9/15; B and B′; arrowheads). If Her6 lacks the Groucho interaction motif WRPW, the construct does not alter neurog1:RFP expression in a control experiment and shows a co-localization of GFP and nuclear-localized RFP (8/12; A and A2; arrowheads). Knock-down of grg1 resembles the her6 morphant phenotype (23/43; D and E) and leads to an increase in the caudal expression of neurog1:RFP (white arrowhead) as well as a decrease of shh:GFP in the MDO (yellow arrowhead) at 30 hpf. Clonal overexpression of her6 and the membrane-bound red lineage marker mCherry in the cTh leads to the activation of the GABAergic rTh marker tal1:GFP (14/21; I; arrowhead). Notably, dividing cells at the ventricular surface are only positive for the mCherry lineage marker (asterisk) but become tal1:GFP positive after radial migration (arrowhead). Cells containing the lineage tracer only do not express Tal1 (H). III, third brain ventricle; cTh, caudal thalamus; PTec, pretectum; PTh, prethalamus; MDO, mid-diencephalic organizer; rTh, rostral thalamus.