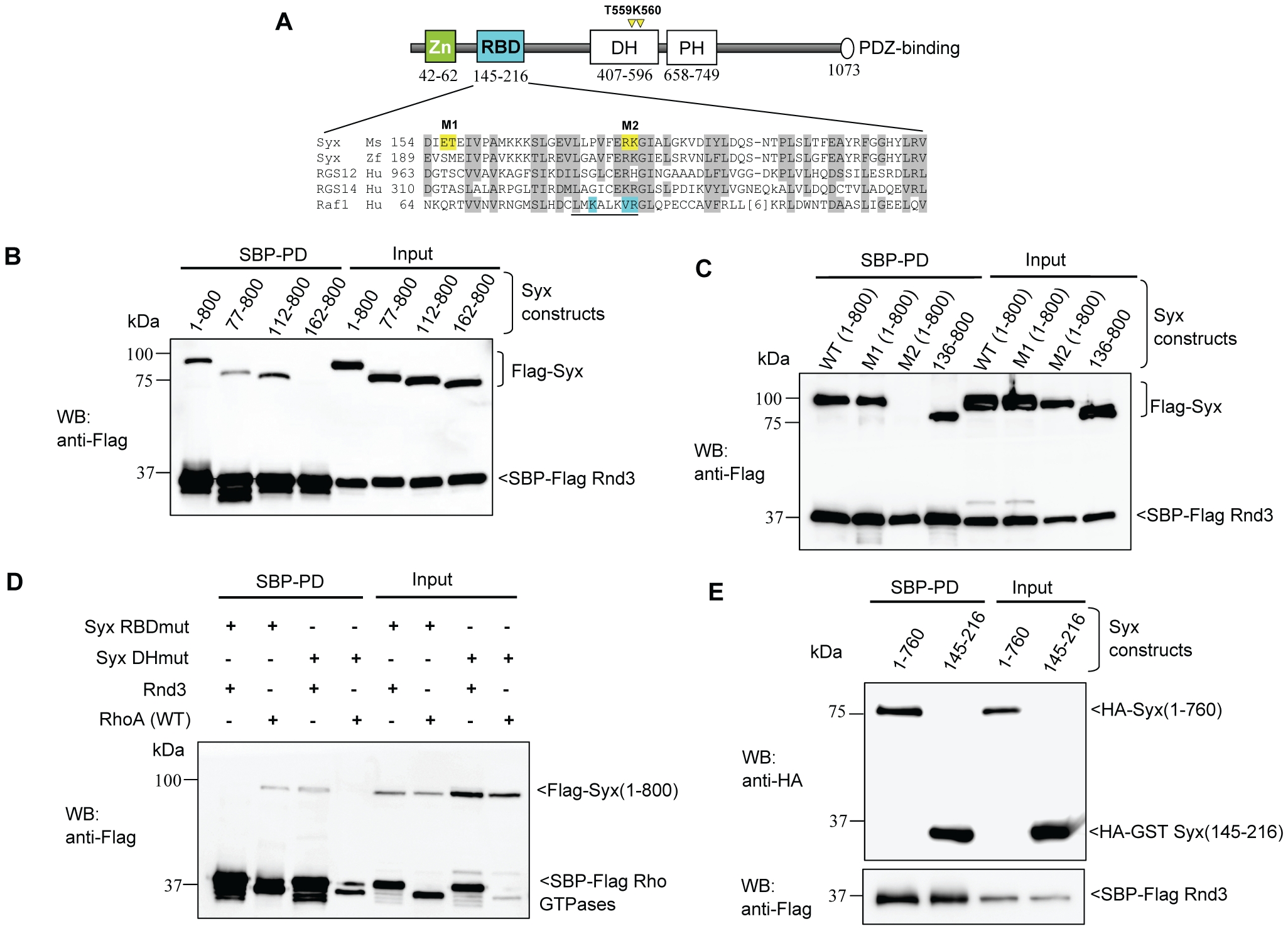
Fig. 3 Characterization of the interaction between Rnd3 and Syx.
(A) Schematic of domains identified in Syx: including zinc-finger, Rnd3-binding (in cyan), Dbl homology (DH), pleckstrin homology (PH) and PDZ-binding motif. Sequence alignment of Raf1-like RBDs in mouse Syx (AAU04953), zebrafish Syx (XM_686228.1), human RGS12 (NP_002917), RGS14 (NP_006471) and Raf1 (AAA60247). Conserved residues are shaded in grey; a key helix is underlined; amino acid side chains contacting Ras are in cyan and residues mutated in this study are in yellow. The catalytic residues T559/K560 in Syx DH domain are denoted by yellow arrowheads. (B) Rnd3 interacts with the N-terminal of Syx. 293T cells were co-transfected with SBP-Flag Rnd3 and indicated Flag-Syx constructs. The purified protein complex was immunoblotted to visualize bound Syx. (C) R178 and K179 of Syx are critical Rnd3 binding residues. 293T cells were co-transfected as for (B) with the indicated constructs and assessed for interaction. M1 and M2 refer to the mutant constructs, E156AT157A and R178EK179D. (D) Rnd3 and RhoA (WT) interact with Syx via the RBD and DH domain, respectively. The mutant constructs, R178EK179D and T559EK560E are represented by Syx RBDmut and Syx DHmut, respectively. (E) Rnd3 interacts with Syx(145–216).