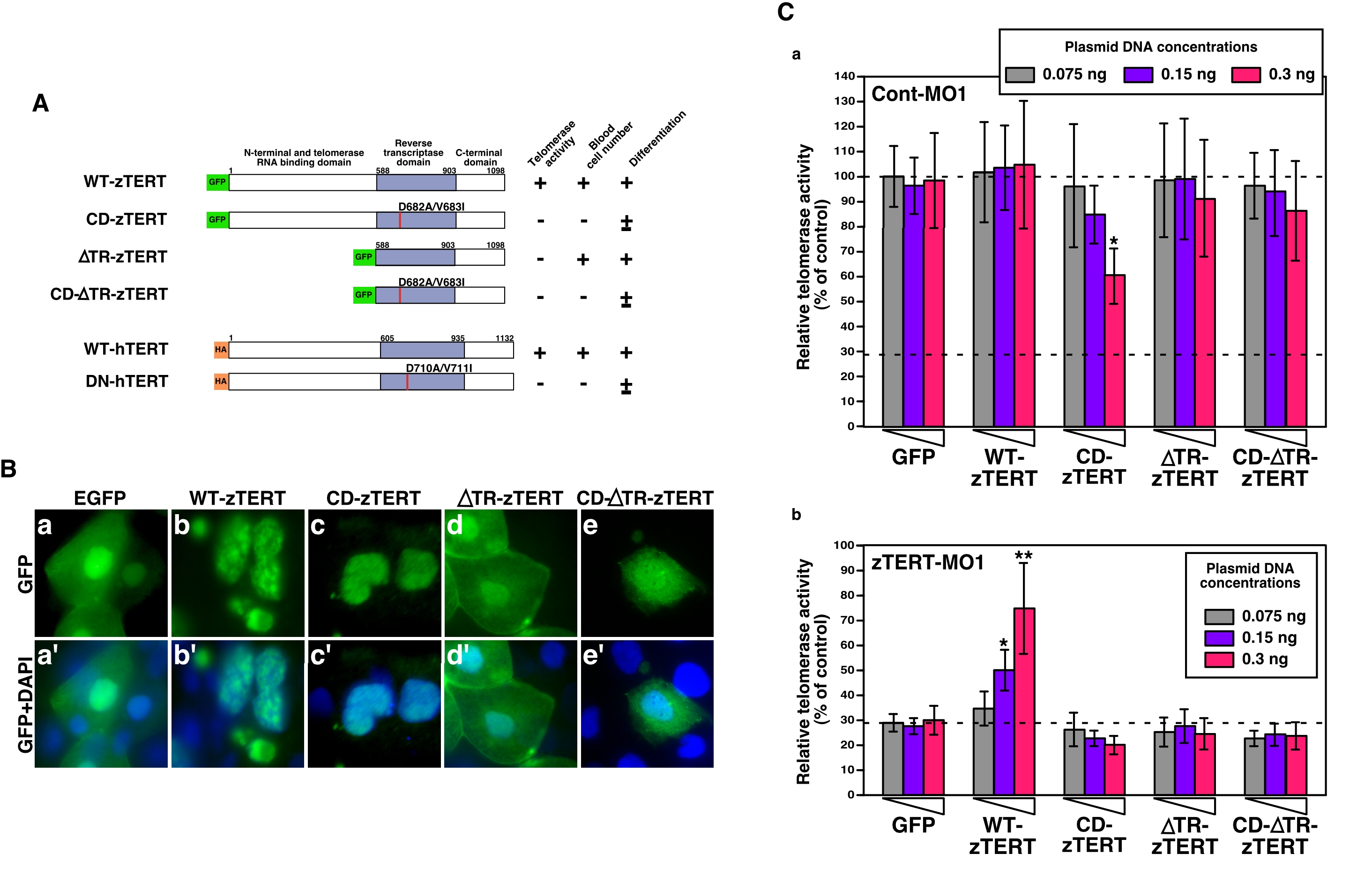
Fig. 6 Expression of zebrafish or human TERT in zTERT-deficient embryos.
(A) Schematic representations of zebrafish and human wild-type TERT and TERT mutants. All zebrafish TERT (zTERT) fragments were tagged with EGFP at their N-termini. The two amino acid substitutions in the RT domain of TERT (see Materials and Methods) are indicated by the red lines. Deletion mutants of the telomerase RNA-binding domain (ΔTR) of zTERT (zTERT-ΔTR) and its amino-acid substitution mutant ΔTR-CD-zTERT were generated. Human TERT (hTERT) and DN-hTERT were tagged with HA at their N-termini. The presence and absence of telomerase activity, detected by TRAP assay, are indicated as + and -, respectively. Significant recovery of blood cell number is indicated by +, and no significant recovery is denoted by -. Full and partial recovery of blood cell differentiation are indicated by + and ±, respectively. (B) Cellular localization of GFP-wild-type (WT) and -mutant zTERT proteins in the zebrafish embryo. GFP is fused to the N-terminus of each TERT protein. The indicated constructs (see Materials and Methods) were injected into zebrafish embryos, and the subcellular localization of the resulting GFP signals was observed at 48 hpf. (a–e) GFP, and (a′–e′) GFP and DAPI. (C) Quantitation of telomerase activity by the expression of zTERT plasmid constructs in zebrafish embryos. Three different concentrations of each zTERT plasmid construct (0.075, 0.15, or 0.3 ng) were co-injected in the indicated combinations with Cont-MO1 (8 ng) (a) or zTERT-MO1 (8 ng) (b), and both the intrinsic and extrinsic telomerase activity was detected by quantitative fluorometric TRAP assay. Each relative telomerase activity value was quantified as a percentage of the activity observed in Cont-MO1-injected embryos expressing a GFP empty vector (GFP) from three independent experiments. *P<0.01, **P<0.001 (Student t-test).